Abstract
Porous microspheres fabricated by biodegradable polymers show great potential as carriers for cell cultivation in tissue engineering. We have attempted to prepare porous microspheres using various biodegradable polymers, and the polymer used is biodegradable Poly(D,L-lactide-co-glycolide) (PLGA), chitosan, hyaluronic acid, gelatin. The influence of fabrication parameters, such as the concentration of crosslinker and polymer, concentration and type of porogen, as well as the post-hydrolysis treatment and thawing time of the porous structure of the microspheres are discussed. an active spread of Hep3B-GFP cells on the microspheres is observed, which indicates that the biodegradable polymeric microsphere with controllable porous structure are of great potential as cell delivery carriers for tissue engineering.
Keywords: Porous Microspheres; PLGA; Chitosan; Hyaluronic Acid; Gelatin
Introduction
Many groups have demonstrated the potential of cell delivery in therapeutic applications by directly injecting a dispersion of cells into the diseased or injured site [1-4]. Despite its proven ability to improve organ and tissue function after damage, cell transplantation is limited by poor graft retention following the delivery of the cells. A significant proportion of the transplanted cells leak out through the hole that is made by the puncturing needle or enter systemic circulation [5,6]. How to precisely control the amount, distribution, and viability of the injected cells has become a major challenge in maximizing the therapeutic effects and elucidating the healing mechanism [7,8].
To prevent loss of cells, various injectable hydrogels, formed from native or synthetic polymers, have been co-implanted with the cells [9-12]. Upon injection, the hydrogels undergo an in-situ sol-gel transition and thus embed the transplanted cells intramuscularly. As a major drawback, the cells encapsulated within the interior of a bulk hydrogel may lack a sufficient supply of oxygen or nutrient, resulting in massive cell death and a non-uniform cell distribution [13]. In addition, their low mechanical strength and durability are still problematic for practical applications [14]. In comparison, porous constructs made of synthetic and/or natural polymers with a three-dimensionally(3D) porous structure are attractive carriers for cell delivery because they allow for quantification of the number of cells injected, as well as improvement of cell viability and retention [15]. In particular, injectable scaffolds show promise for this application, as cells can be mixed homogeneously with the scaffold formulation prior to injection. The ability to deliver injectable scaffolds in a minimally invasive manner to a cavity of any size or shape renders them especially attractive for clinical use in tissue repair [16]. One type of injectable scaffold for tissue engineering applications involves the use of discreet polymer microspheres. Microspheres can be fabricated using a variety of different biodegradable polymers such as chitosan, gelatin and PLGA, and their use for delivery of cells and growth factors for repair of tissues such as bone, skin and brain has been reported [17-21]. To establish a polymer and porosity that would produce structurally stable porous scaffolds, we tried to manufacture microspheres from various polymers. Finally, an in vitro Hep3BGFP cell culture on the porous microspheres was carried out to evaluate the influence of the various polymer microspheres on cell growth and to assess the microspheres biomedical potentials as microcarriers.
Experimental
Materials
Poly(D,L-lactide-co-glycolide) (PLGA, 75:25, Mw~106000) was purchased from Birmingham Polymers (Pelham, AL, USA). Gelatin (type A, from porcine skin), PVA (Mw13 000–23 000, 98% hydrolyzed), Dichlormethane (DCM, 99%), chitosan (low molecular weight), and acetic acid were purchased from Sigma- Aldrich® (St. Louis, MO). Hyaluronic acid (viscosity: 1.35cm2/kg) was purchased from HUMEDIX (KR). Hep3B- GFP cells were kindly provided by the NCEED of inha university hospital. Medium, Trypsin-EDTA, and PBS for cell culture were purchased from Gibco (Invitrogen, Carlsbad, CA, USA). All other reagents were of analytical grade.
Preparation of Porous PLGA Microspheres by Gas-Foaming Method
Gas-foaming method was used with the ammonium bicarbonate as porogen. To 8 mL of methylene chloride containing 6.25% (w/v) PLGA, 2.5 mL of deionized water containing different amounts of ammonium bicarbonate (5, 10, 20 and 30%, w/v) was added. The first W-O emulsion was prepared using a homogenizer (Dispenser T 10 basic, IKA Works, Inc.) at 5,000 rpm for 3 min. This primary emulsion was immediately poured into a beaker containing 300 mL of 0.1% (w/v) PVA solution and then was re-emulsified by using a magnetic stirrer (HS 10, IKA Works, Inc.) for 4 hours at 200 rpm. After the solvent was evaporated, the microspheres were separated by centrifugation, washed three times with distilled water, and lyophilized using a freeze dryer [22]. The lyophilized microspheres were immersed in 80 mL of various concentrations of NaOH solution for 2 hours for control of pore size. Afterward, the hydrolyzed microspheres were washed with distilled water to remove the remaining NaOH solution [23].
Preparation of Porous PLGA Microspheres by Microfluidic System
On the other hand, porous PLGA microspheres were prepared using a microfluidic system that consisted of a Tygon® tube (0.8 mm i.d. 1/16 in. o.d.), a glass capillary (1.15 mm i.d. 1.5 mm o.d.), and needles (25G). The device with two-way flow channels was fabricated by inserting the needle and the capillary tube into the Tygon® tube, followed by sealing with epoxy adhesive [24]. The W-O emulsion was prepared by emulsifying a 7.5% aqueous solution of gelatin (w/v, 2g) and 1% PVA (w/v) in a 2% PLGA solution (w/v, 6g) with a homogenizer (Dispenser T 10 basic, IKA Works, Inc.) at 20,000 rpm for 3 min. Subsequently, the prepared emulsion was introduced as a discontinuous phase into the fluidic device, in which 1% aqueous PVA solution (w/v) served as the continuous phase. The flow rates of the discontinuous and continuous phases were maintained at 0.05 and 2 mL/min. by syringe pump (LSP01, Longer pump) and peristaltic pump (Matesrflex p/s, THERMO), respectively. The W-O-W droplets, formed at the tip of the needle, flowed along the capillary tube into ice-cold water (collection phase) and were gently stirred overnight to remove the organic solvent by evaporation. To remove the residual gelatin, the acquired microspheres were gently stirred for 3 hours in a warm water bath. The resultant microspheres were washed with deionized water three times and collected for further application [25].
Preparation of Porous Chitosan Microspheres
Porous chitosan microspheres were prepared using freezedrying method by the encapsulator (B-395 PRO, Buchi). The setup consisted of a syringe pump, a 10 mL syringe with 80 um nozzle, and a Dewar flask (KGW). A series of chitosan solutions (1, 2 and 4%, w/v) in 100 mM acetic acid was used. The positive lead from the power supply was attached to the nozzle, and the collector (Dewar flask) was connected to the ground. The distance between the needle tip and the collector was 5 cm. With 1300 Hz frequency and electrode 1,000 V applied by a power supply, the chitosan solution was continuously pumped out through the needle by the syringe pump at a flow rate of 1.5 mL/min., forming microspheres in a dripping mode, and collected into a Dewar flask filled with liquid nitrogen. To investigate the control of pore size, the collected microspheres were thawed through shaking for different periods of time in the Dewar flask [26]. Aliquots of the sample for scanning electron microscopy were then lyophilized (FreeZone® 4.5 ©Labconco freeze dry system) for 2 days.
Preparation of Porous Hyaluronic Acid Microspheres
Porous hyaluronic acid microspheres were prepared according to the procedure that was described for the fabrication of chitosan. A series of hyaluronic acid solutions (1, 2 and 4%, w/v) in distilled water was used. To investigate the control of pore size, the lyophilized hyaluronic acid microspheres were stirred in ethanol of various concentrations (0.5, 10, 20 and 30% crosslink yield) of cross-linking agent for 24 hours. After the cross-linking agent was removed, the microspheres were separated by centrifugation and re-lyophilized using a freeze dryer.
Preparation of Porous Gelatin Microspheres
Porous gelatin microspheres were prepared according to the procedure that was described for the fabrication of chitosan. A gelatin solution (20%, w/v) in distilled water was used. Crosslinking was carried out by immersing the microspheres in glutaraldehyde solution and stirred for 12 h at 4℃. To investigate the control of pore size, the gelatin microspheres were freezing at various temperatures (-20, -40 and -70℃). After freezing, the microspheres were separated by centrifugation and re-lyophilized using a freeze dryer.
Characterization of Microspheres
Scanning electron microscopy (SNE-4000M, SEC, KR) was used to observe the surface morphology and pore size of the microspheres. The morphology of microspheres was observed after gold coating using a sputter- coater (MCM-100, SEC, KR). The distribution of pore and microspheres size calculated using ImageJ.
Cell Culture onto Porous Microspheres
The various porous microspheres were sterilized by 70% ethanol solution, washed with phosphate-buffered saline, and preincubated overnight in the culture medium at 37℃, respectively. To seed the cells, 1x106 dissociated Hep3B-GFP cells and roughly 200 porous microspheres were suspended in a 60 mL culture medium in a spinner flask (INTEGRA, NH, USA) and stirred at 50 rpm. One week after culture, the cell-loaded microspheres were examined under a fluorescent microscope to analyze cell adhesion on the microspheres. The number of cells on the porous microspheres was determined on 7days by measuring the CellTiter-Glo® Luminescent Cell Viability Assay [27].
Results and Discussion
Preparation of Porous PLGA Microspheres
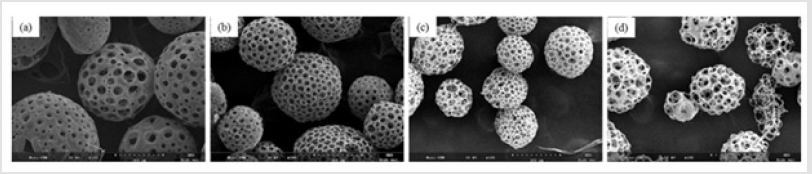
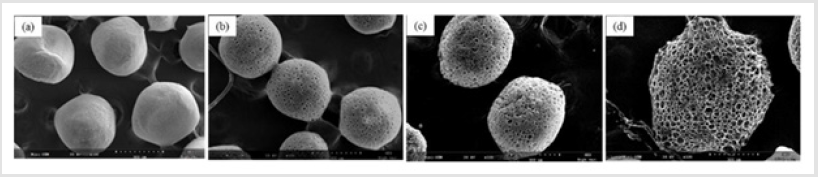
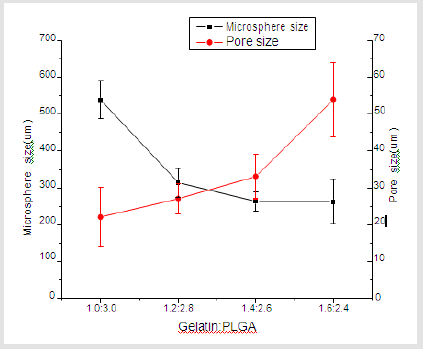
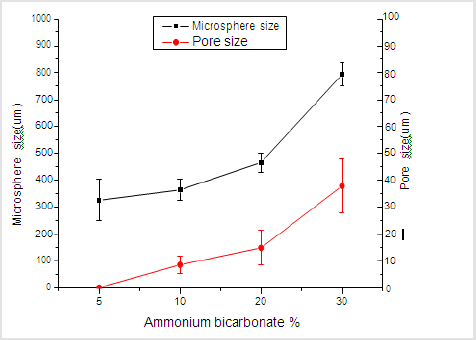
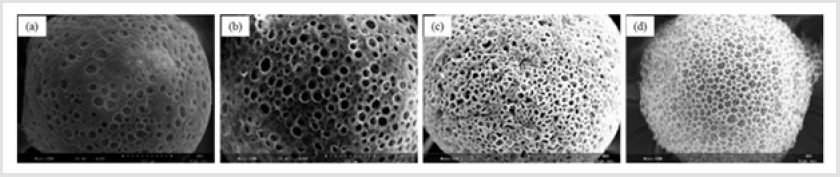
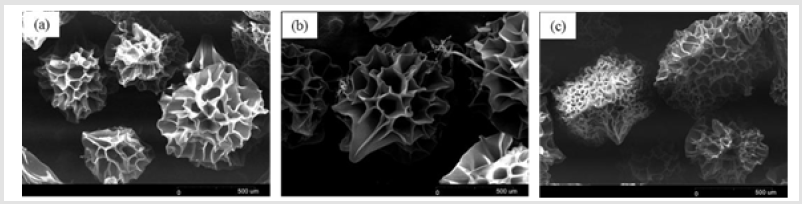
Figure 1 shows that the homogeneity of microspheres became very porous with the increase of gelatin ratio in the microspheres. As the PLGA concentration decreased from 3.0 to 2.4 mass ratio, the diameter of the porous microspheres decreased from 220-330μm to 480-590μm while the pore size increased from 12-30μm to 50- 60μm. In addition, as shown in Figure 2, morphologies of porous PLGA microspheres were prepared by incorporating different amounts of ammonium bicarbonate in the W1 aqueous phase. As the ammonium bicarbonate amount increased from 5 to 30 weight %, the diameter of the porous Figures 3 & 4 microspheres increased from 250-400μm to 750-840 μm while the pore size increased from less than 1μm to 27-48μm. By increasing the amount of ammonium bicarbonate, a more porous structure on the surface could be attained. PLGA concentration that is too low results in frail, membrane-like microspheres with low mechanical strength, which indicates the instability of the PLGA microspheres fabricated under this condition. Figure 5 shows the morphologies of microspheres before and after hydrolysis. After hydrolysis in NaOH solution, a very homogeneous, porous structure in both the surface and interior of the microspheres was formed. Both the microspheres’ size and porosity could be tuned by changing the concentration of a polymer. An increase of polymer concentration led to an increase in bead size and reduction in pore size because the viscosity of the polymer solution increased with the polymer concentration, restricting expansion of the bubbles in the polymer solution. In addition, the sizes of both beads and pores could be adjusted by changing the amount of the porogen added into the system. An increase in the concentration of porogen increased the average diameter of the beads and the surface pore size, although this method is simple and easy to use [22]. After hydrolysis in NaOH solution for the porosity control of the PLGA microspheres, a very homogeneous, porous structure in both the surface and interior of the microspheres was formed, increasing the concentration of NaOH. This indicates that the hydrolytic treatment mainly changes the porous structure of the surface layer of the microspheres. Therefore, the hydrolysis technique could be used to fabricate porous microspheres with homogeneous and adjustable porous structures from the surface to the interior. Furthermore, hydrolysis treatment provides a good method to modify the surface properties of each pore of the microspheres [23].
Figure 1: SEM images of porous PLGA microspheres prepared by microfluidic system method containing different mass ratio of PLGA and gelatin. (a) 3.1; (b) 2.8:1.2; (c) 2.6:1.4;(d) 2.4:1.6.
Figure 2: SEM images of porous PLGA microspheres prepared by W/O/W double emulsion method containing different amounts of ammonium bicarbonate in the W1 phase. (a) 5%; (b)10%; (c) 20%; (d) 30%w/v.
Figure 5: SEM images of porous PLGA microspheres before and after hydrolysis in different concentration of NaOH solution. (a) Original PLGA; (b) 0.1M; (c) 0.15M; (d) 0.2M.
Preparation of Porous Chitosan Microspheres
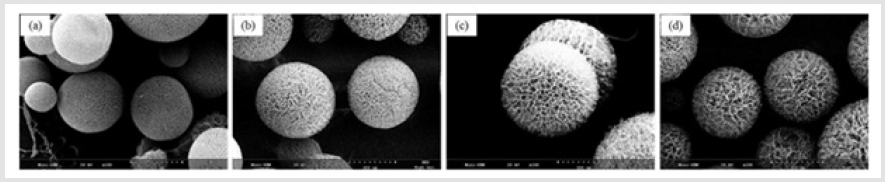
Figure 6 shows the morphologies of porous chitosan microspheres obtained; that the pores were well interconnected; and that their distribution was fairly homogeneous. As the chitosan concentration increased from 1 to 4 wt%, the diameter of the porous microspheres was increased and the pore size and porosity were decreased. As shown in Figure 7, the pore size was dramatically increased by the thaw-refreeze method. It was evident that a longer thawing time results in the formation of larger interconnected pores inside the microspheres. When the chitosan microspheres were frozen with liquid nitrogen, the water crystallized into ice rapidly, which resulted in a small pore size. However, when the frozen chitosan microspheres were slightly thawed under specific conditions, the melted ice crystals and chitosan molecules restored the mobility, combined with each other, and finally reformed their structure [26].
Figure 6: SEM images of porous chitosan microspheres prepared by freeze drying method containing different amounts of chitosan. (a) 1%; (b) 2%; (c) 4%w/v.
Figure 7: SEM images of porous chitosan microspheres prepared by freeze-drying method in different thawing time. (a)original chitosan; (b)10min; (c) 30min; (d) 45min.
Preparation of Porous Hyaluronic Acid Microspheres
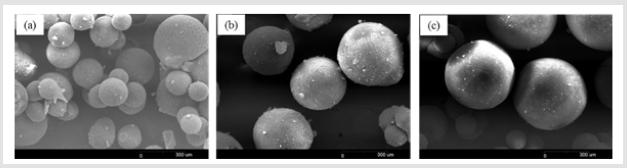
As shown in Figure 8, hyaluronic acid microspheres prepared from varying concentrations of hyaluronic acid and were observe their morphological features. The porous structure of hyaluronic acid microspheres became dense with an increase in the hyaluronic acid concentration. As shown in Figure 9, the cross-linking agent concentration of the initial precursor solution affected the porosity of the resulting microsphere. Microspheres were not formed when the concentration of cross-linking agent was 5% or less. the pore size control of the water-soluble polymer as hyaluronic acid could be tuned by changing the concentration of cross-linking agent. Covalent linkages between polymer chains were obtained by the reaction of functional groups of a cross- linking agent (Epoxy group) and HA (hydroxyl group). It is known that the porous network can be adjusted by the amount of cross-linking agent [28].
Figure 8: SEM images of porous hyaluronic acid microspheres prepared by freeze-drying method containing different amounts of hyaluronic acid. (a) 10%; (b) 20%; (c) 30%w/v.
Figure 9: SEM images of porous hyaluronic acid microspheres prepared by freeze-drying method containing different concentration of crosslinking agent. (a) 0.5%; (a) 10%; (b) 20%; (c) 30% (crosslink yield).
Preparation of Porous Gelatin Microspheres
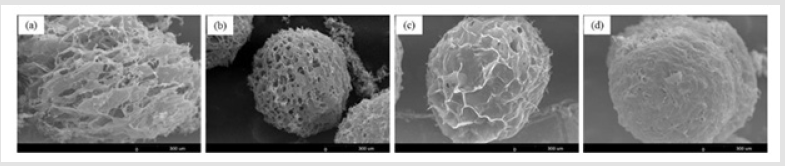
The SEM images of the obtained porous gelatin microspheres are shown in Figure 10. It can be found that the microspheres have an interconnected porous structure. The pore size range was 20-80 μm, which is suitable for cell attachment. The free water in the gelatin microspheres was frozen, which caused the polymer chains to gather and condense. The mean pore diameter could be controlled by varying the freezing temperature which is related to the cooling rate [29]. since the ice crystal growth rate and the pore diameters are functions of the temperature gradient. The pore size of gelatin microspheres decreased with freezing temperature for - 80℃. The free water in the gelatin microspheres was frozen more densely at low temperatures, which caused the polymer chains to gather and condense.
Figure 10: SEM images of porous gelatin microspheres prepared by freeze-drying method with different freezing temperature. (a) -20; (b) -40; (c) -80℃.
Cell Culture onto Porous Microspheres
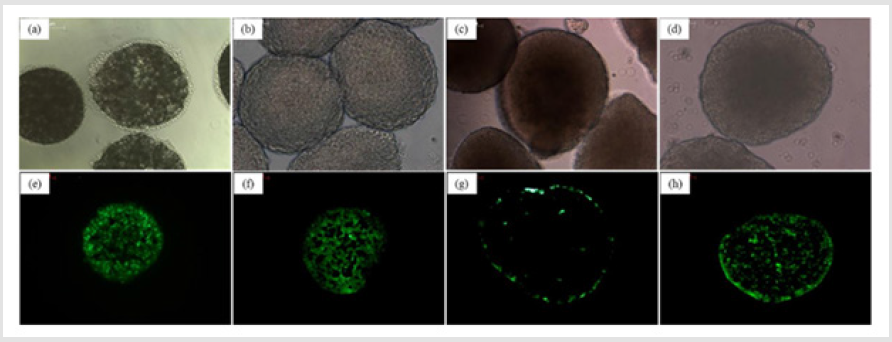
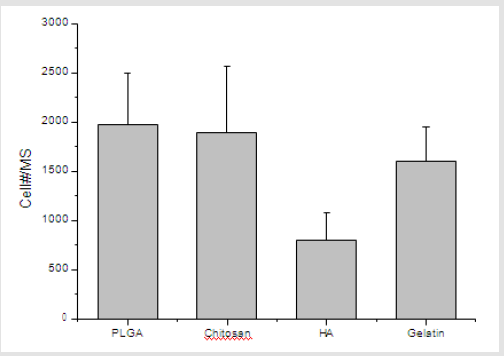
To evaluate the efficiency of cell seeding onto porous microspheres formed from the various polymer microspheres, the fluorescence images of Heb3B-GFP attached to the porous microspheres were observed after 7 days of seeding. As time elapsed, the inoculated cells continued to migrate, proliferate, and eventually spread throughout the microspheres. After 7 days, it was found that most cells attached uniformly to the surface and cross section of the microspheres except hyaluronic acid (Figure 11). In the case of hyaluronic acid, low crosslink-yield induced swelling under cell culture conditions and high crosslink-yield reduced pore size. As shown in Figure 12, cell viability of cell culture onto porous microspheres confirmed the high cell viability in PLGA, chitosan and gelatin microspheres. The difference in cell viability can be explained as follows. Some of the pores in the hyaluronic acid microspheres were blocked by swelling in media. This would lead to a limited transportation of nutrients/oxygen and eventually cell death in the central region of the microspheres [30].
Figure 11: Optical micrographs (Whole body) and fluorescence images (cross-section) of porous microspheres cultured with Hep3B-GFP. (a,e) PLGA; (b,f) Chitosan; (c,g) Hyaluronic acid; (d,h) Gelatin.
Figure 12: The number of Hep3B-GFP adherent to cell-loaded microspheres in spinner flask culture after 7days.
Conclusion
In this study, porous microspheres were used to ensure good retention of the engrafted cells as a platform for cell delivery. The pore size could be controlled, depending on the polymer and method of preparation. This study demonstrates the feasibility of using porous microspheres as a cell culture substrate to expand cells and as a cell transplantation vehicle. These findings may contribute to the development of injectable tissue engineering solutions for therapy and repair of tissue.
References
- Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP (1997) Osteogenic differentiation of purified, culture‐expanded human mesenchymal stem cells in vitro. Journal of Cellular Biochemistry 64: 295-312.
- Bruder SP, Fox BS (1999) Clinical Orthopaedics and Related Research, 367 Suppl, S68.
- Patrick CW (2001) Tissue engineering strategies for adipose tissue repair. The Anatomical Record 263: 361-366.
- Patrick CW (2000) Adipose tissue engineering: The future of breast and soft tissue reconstruction following tumor resection. Semin in Surgical Oncology 19: 302-311.
- Berardis S, Sattwika PD, Najimi M, Sok EM (2015) Use of mesenchymal stem cells to treat liver fibrosis: Current situation and future prospects. J World Gastroenterol 21: 742-758.
- Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, et al. (2010) Circulation Research 106: 1570.
- Dupont KM, Sharma K, Stevens HY, Boerckel JD, Garcia AJ, et al. (2010) Human stem cell delivery for treatment of large segmental bone defects. Proc Proceedings of the National Academy of Sciences 107: 3305-3310.
- Redenti S, Neeley WL, Rompani S, Saigal S, Yang Y, et al. (2009) Engineering retinal progenitor cell and scrollable poly(glycerol-sebacate) composites for expansion and subretinal transplantation. Biomaterials 30: 3405-3414.
- Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, et al. (2011) A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials 32(30): 7514-7523.
- Ye Z, Zhou Y, Cai H, Tan W (2010) Advanced Drug Delivery Reviews 63: 688.
- Miyagi Y, Zeng F, Huang X-P, Foltz WD, Wu J, et al. (2010) Surgical ventricular restoration with a cell- and cytokine-seeded biodegradable scaffold. Biomaterials 31: 7684-7694.
- Ooi HW, Hafeez S, van Blitterswijk CA, Moroni L, Baker MB (2017) Hydrogels that listen to cells: A review of cell-responsive strategies in biomaterial design for tissue regeneration. Materials Horizons 4: 1020-1040.
- Demol J, Lambrechts D, Geris L, Schrooten J, Van Oosterwyck H (2011) Biomaterials 32(1): 107-118.
- Hong Y, Gong Y, Gao C, Shen J (2008) Collagen‐coated polylactide microcarriers/chitosan hydrogel composite: Injectable scaffold for cartilage regeneration. Journal of Biomedical Materials Research 85: 628-637.
- Choi SW, Zhang Y, Yeh YC, Wootena AL, Xia Y (2012) Biodegradable porous beads and their potential applications in regenerative medicine. J Mater Chem 22: 11442-11451.
- Qutachi O, Vetsch JR, Gill D, Cox H, Scurr DJ, et al. (2014) Injectable porous microspheres form highly porous scaffolds after injection. Acta Biomaterialia 10: 5090-5098.
- Bhat A, Dreifke MB, Kandimalla Y, Gomez C, Ebraheim NA, et al. (2010) J Tissue Eng Regen Med 4: 532.
- Huang L, Xiao L, Poudel AJ, Li J (2018) Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydrate Polymers 202: 611-620.
- Bible E, Chau DY, Alexander MR, Price J, Shakesheff KM, et al. (2009) The support of neural stem cells transplanted into stroke-induced brain cavities by PLGA particles. Biomaterials 30(16): 2985-2994.
- Bible E, Qutachi O, Chau DY, Alexander MR, Shakesheff KM, et al. (2012) Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials 33(30): 7435-7446.
- Kawai K, Suzuki S, Tabata Y, Ikada Y, Nishimura Y (2000) Accelerated tissue regeneration through incorporation of basic fibroblast growth factor-impregnated gelatin microspheres into artificial dermis. Biomaterials 21(5): 489-499.
- TK Kim, JJ Yoon, DS Lee, TG Park (2006) Gas foamed open porous biodegradable polymeric microspheres. Biomaterials 27(2): 152-159.
- Shi X, Sun L, Jiang J, Zhang X, Ding W, et al. (2009) Biodegradable Polymeric Microcarriers with Controllable Porous Structure for Tissue Engineering. Macromolecular Bioscience 9: 1211-1218.
- Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF (1999) Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials 20: 573-588.
- Choi SW, Yeh YC, Zhang Y, Sung HW, Xia Y (2010) Uniform Beads with Controllable Pore Sizes for Biomedical Applications. small 6: 1492-1498.
- Maeng YJ, Choi SW, Kim HO, Kim JH (2010) Culture of human mesenchymal stem cells using electrosprayed porous chitosan microbeads. Journal of Biomedical Materials Research 92(3): 869-876.
- Ryu JH, Kim SS, Cho SW, Choi CY, Kim BS, et al. (2004) Novel bioactive and biodegradable glass ceramics with high mechanical strength in the CaO-SiO2-B2O3 Journal of Biomedical Materials Research 68(1): 79-89.
- Kim JT, Lee DY, Kim TH, Song YS, Cho NI (2014) Biocompatibility of hyaluronic acid hydrogels prepared by porous hyaluronic acid microbeads. Metals and Materials International 20: 555-563.
- Nam YS, Park TG (1999) Biodegradable polymeric microcellular foams by modified thermally induced phase separation method. Biomaterials 20: 1783-1790.
- Choi SW, Yeh YC, Zhang Y, Sung HW, Xia Y (2010) Uniform Beads with Controllable Pore Sizes for Biomedical Applications. small 6(14): 1492-1498.

 Research Article
Research Article