Abstract
Mycotoxins are secondary metabolites produced by fungal species. Detoxification processes of plants which are infected with the toxigenic fungi are responsible for the conversion of them to mycotoxin derivatives, called masked mycotoxins. Masked mycotoxins are less toxic metabolites for plants according to their free forms. Both of these metabolites have toxic effects on animals and humans. Masked mycotoxins are hydrolyzed into their free forms by human and animal intestinal microbiomes. Here, we reviewed the unforeseen toxicity of masked trichothecenes, especially focused on deoxynivalenol (DON) and zearalenone (ZEN), which are classified as major mycotoxins of Fusarium species.
Keywords: Masked Mycotoxins; Toxicity; Fusarium; Zearalenone (ZEN); Deoxynivalenol (DON)
-->Introduction
Since ancient times the importance of food in human health has been known and their relationship was highlighted by Hippocrates in the 5th Century BC with the statement “Let thy food be thy medicine and medicine be thy food” [1]. Every year approximately a million people experience a foodborne illness. Food contaminated with microorganisms and/or their chemicals, leads to these illnesses and becomes a burden on public health [2]. Among the main food toxicants, mycotoxins are secondary metabolites, produced by various fungal organisms. Aflatoxin, ochratoxin A, citrinin, patulin, trichothecenes, fumonisins, and zearalenone (ZEN) are the most common mycotoxins synthesized by Aspergillus, Fusarium, Penicillium, Alternaria species [3- 5]. Mycotoxin contamination can occur at all stages from the cultivation of plants in the field to various production steps - such as processing, distribution, storage, preparation - of foods and agricultural products [2,6,7]. Cereals are the basic source of the human and animal diet. Fusarium graminearum and F. culmorum are the most prevalent phytopathogenic species that cause epidemics such as Fusarium Head Blight (FHB), crown and root rot diseases in small-grain cereals, especially wheat, barley, and maize. Mycotoxin contamination in the cereals lead to reduction in crop quality and quantity and resulted in economic losses around the World [8-10]. Class B-trichothecenes and ZEN are included in the major groups of Fusarium mycotoxins [11,12]. Class B-trichothecenes comprise deoxynivalenol (DON), nivalenol (NIV) and their acetylated derivatives (15-acetylated deoxynivalenol (15- ADON) and 3-acetylated deoxynivalenol (3-ADON), 4-acetylated nivalenol (4-ANIV)). However, DON is the most accumulated class-B trichothecene by cereals [6,13-18].
Masked forms of DON and ZEN, and their Toxicity
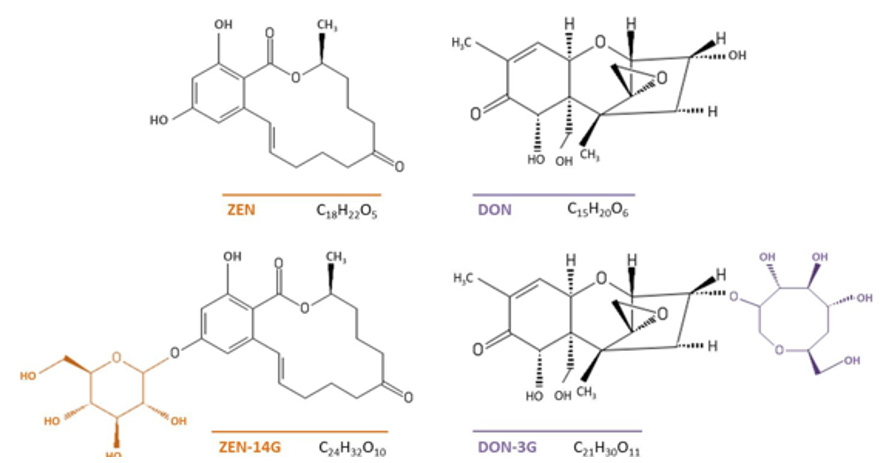
Masked mycotoxins are generated as a result of the transformation of the main type of mycotoxins by living plants in order to protect themselves from xenobiotic compounds [19]. They were detected in early 1990 and termed as masked due to the inability to be screened in food and feed through conventional analytical methods [20]. The most frequently detected masked forms of Fusarium toxins are deoxynivalenol-3-glucoside (DON-3G) and zearalenone-14-D-glucopyranoside (ZEN-14G). The isolation of DON-3G was first reported from naturally contaminated maize and wheat [21]. Then, the DON-3G also has been found in barley, oat and cereal-based food and feeds [22-25]. The toxicity of DON is provided by three free hydroxyl groups (-OH). DON is converted into DON-3G as a result of glucose transferring to its hydroxyl group at carbon 3 [6,26]. ZEN-14G is another common masked mycotoxin that has occurred in naturally infected cereals such as wheat, barley and maize. ZEN is reduced to phase I metabolites named α-zearalenol (α-ZEL) and β-zearalenol (β-ZEL). The glucose conjugates of these compounds are also produced (Figure 1).
Figure 1: Structures and molecular formulas of ZEN, DON, and their masked forms, ZEN-14G and DON-3G.
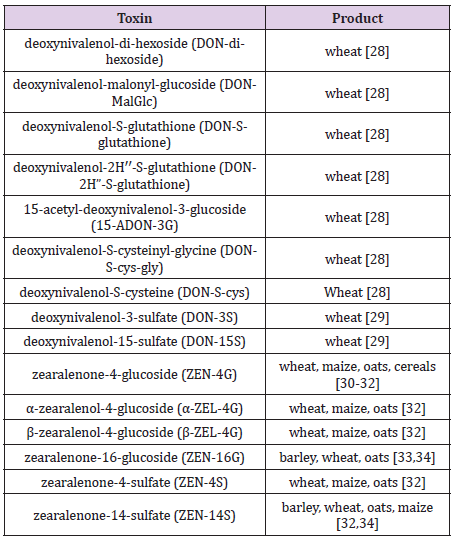
Transformation of DON, ZEN and their metabolites with glucose, glutathione, and sulfate groups were listed in Table 1. In addition to masked forms, the modified forms of mycotoxins could occur by transformation or degradation of mycotoxins during food processing and the production of industrial goods. Due to their stable structure and features, mycotoxins tend to remain in the ultimate products [5]. While all these forms can be called as hidden, conjugated, and bound, the term masked is used only for plant metabolites [3,12,27-34].
Mycotoxins have various effects like mutagenic, teratogenic, carcinogenic on eukaryotic organisms [5,35,36]. Epidemiological cases were reported in different regions of Asia (Japan, Korea, China, India) related to the consumption of contaminated cereals and cereal-based foods. The population affected by DON caused outbreaks to trigger especially gastrointestinal disorders in the population [37-39]. DON can cause anorexia, growth retardation, immune dysregulation, altering neuroendocrine signaling, proinflammatory gene induction, and disruption of the growth hormone axis. At the cellular level, DON can inhibit protein synthesis; can disrupt cell signaling, differentiation and proliferation; and even can cause death [40-42]. ZEN, on the other hand, has a toxic effect on the endocrine and reproductive system and also shows developmental toxicity. Due to its estrogenic activity, it most affects the reproductive organs and their functions [43,44]. The natural and artificial DON and ZEN intakes are resulted in accumulation of these mycotoxins in organs, tissues and biofluids of animals and humans. DON is detected in urine, ZEN in the endometrium, ZEN and α-ZEL in the blood of humans, whereas DON, ZEN, and their metabolites can be found in almost all animals’ tissues and organs. Interestingly, these mycotoxins also appear in hen eggs, cow and sheep milks, chicken meat, fish [45,46], which are consumed as a food. Therefore, people are exposed to mycotoxins not only through plants but also through animals.
In addition to main forms, masked endotoxins co-occur as contamination agents of foods and feeds. Masked mycotoxins exhibit lower toxicity than their free forms. This attenuated toxicity of masked forms was demonstrated in vitro in mammalian cell lines. Distinct intestinal epithelial (the piglet IPEC-J2, the human Caco-2) cell lines were treated with DON and DON-3G, it was detected that DON reduced cell viability, whereas DON-3G showed no cytotoxic effect on these cell lines. Moreover, non-cytotoxic concentration of DON induced a significant decrease in trans-epithelial electrical resistance (TEER), by contrast DON-3G did not decrease TEER. In addition, differentiated cells were more resistant to DON and DON- 3G than the proliferative cells [47,48]. It was also confirmed that DON-3G displayed no cytotoxic effect on human gastric epithelium (GES-1) cells [49]. Likewise, the viability of Caco-2 cells, exposed to ZEN and to its masked forms, was not significantly affected. Intestinal transfer of two glucosylated masked forms of ZEN (ZEN- 14G and ZEN-16G) revealed that intestinal cells took these masked forms up, and then ZEN was released by enzymatic cleavage [50,51]. ZEN-14G showed no effect on human breast cancer (MCF- 7) cells. Also, these cells converted of ZEN-14G to several common aglycones with xenoestrogen activity and all of these metabolites ultimately occasioned the toxic impact [1].
Besides, in vitro toxicity studies were conducted through imitating organism’s fluids [52]. Artificial digestive juices (saliva, gastric juice, duodenal juice, bile) exhibited no capacity to hydrolyze masked forms (DON-3G, ZEN-14G, and ZEN-14S) of Fusarium mycotoxins. Acidic hydrolysis and enzymatic simulations of lower gastrointestinal (GI) tract also showed no ability to regenerate main forms [53-57]. It was demonstrated that DON-3G was not hydrolyzed in the stomach of mammals and also by human cytosolic glucosidases. Fungal cellulase and cellobiose preparations carry out the partial cleavage, while several lactic acid bacteria such as Lactobacillus plantarum, Enterococcus durians, Enterococcus Mundie have significant capacity to hydrolyze of DON-3G (in vitro) [53]. Thus, specific intestinal microbiomes consisting of various bacterial species are able to transform masked mycotoxins to their parent forms [58]. The investigation of ZEN-14G metabolism in liver microsomes (in vitro) of several animals (rats, chickens, swine, goats, cows) and humans indicated that the hydrolysis of the masked form occurred via the main metabolic pathway, which include reduction, hydroxylation and glucuronic acid (GlcA) conjugation [59]. Human’s fecal microbiota (in vitro) are able to deconjugate masked mycotoxins DON-3G, ZEN-14G, ZEN-14S, and thereby to release toxic aglycones and generate unspecified catabolites. The released main mycotoxins, as a result of masked forms transformation, lead to an increase in the toxic effects in exposed individuals. For this reason, masked mycotoxins should be also included in the consideration during the evaluation of the population exposed to mycotoxin [55,60].
The intrinsic toxicity study revealed that oral uptake of DON- 3G in rats was hardly hydrolyzed in the stomach, whereas ZEN-14G was rather unstable in the stomach and formed the main form ZEN. The intense hydrolysis of DON-3G, ZEA-14G to their main forms was detected in the intestine [61]. Studies on pigs (in vivo) related to conversion of masked mycotoxins (DON-3G) to their main forms indicated the hydrolyzing of DON-3G in the distal small and large intestine [62]. Likewise, it was exhibited that this masked form was cleaved during digestion in rats and pigs (in vivo), and recovered as DON, which subsequently excreted in feces and urine [63,64]. Additionally, oral uptake of ZEN-14G and ZEN-16G in piglets showed that these masked forms were converted in the digestive tract to the ZEN, which then was detected in feces and urine [65]. After intravenous and oral administration of α-ZEL and α-ZEL-14G on rats’ plasma, a large extent of ZEN, α-ZEL and GlcA conjugates detected as a result of transformation. Despite the fact that the oral bioavailability was exceedingly low, ZEN, α-ZEL, ZEN-14G are able to instigate toxicity after hydrolysis. Consequently, low oral bioavailability of masked mycotoxins does not have a correlation with limited toxicity [59,66]. Although earlier findings showed the lower toxicological relevance of masked forms compared to main mycotoxins, recent studies with intravenous and oral administration highlighted the systemic exposure of organisms depending on the releasing considerable level of main forms [67]. As can be understood from these examples, the intestinal microbiome plays a major role in the chemical modification of masked mycotoxins also in humans. In conclusion, masked forms of Fusarium mycotoxins can be reconstructed to free forms during digestion and thus potentially increase the risk of these forms for humans and animals. Considering all of these risks, the Panel on Contaminants in the Food Chain of the European Food Safety Authority concluded that the modified forms of mycotoxin must be valued with the similar toxicity of its main form [58,68,69].
Conclusion
Food safety has great importance in the maintenance of human health and wellbeing. For this reason, it is essential to prevent the contamination with DON and ZEN, the foodborne Fusarium endotoxins, which is a major concern all over the world. Originating from this point, we tried to highlight the different toxic effects of these two major toxins and their masked forms by comparing the in vitro and in vivo studies. Although in vitro studies show that masked forms have a lower toxic effect on animal and human cells than main mycotoxins, in vivo studies have demonstrated that masked forms have significant toxicity due to their conversion to the free form by enzymatic reactions. Since these toxins cause losses in agricultural production and various human health problems, unexpected toxicity of masked forms should be considered. Therefore, identification of toxin types and its masked forms in agricultural products and then measurement of the exposure to them by using validated biomarker analysis or PCR-based approaches have great importance for the risk management. The Fusarium outbreaks which strongly correlated to climate change lead to inevitably spreading of its mycotoxin in food and feed. Moreover, transporting agricultural goods causes the spreading of Fusarium species, and the increase in contaminated human and animal numbers by these secondary metabolites, which previously influence in definite regions. In agricultural areas, levels of mycotoxins and their masked forms should be screened routinely and continuously with rapid diagnostic kits. However, the most important approach for controlling mycotoxins and their harmful effects is fighting against Fusarium species in agricultural areas. In this context, various strategies, such as breeding of Fusarium resistant plant species, crop rotation and using synthetic or natural fungicides, have been used for the management of Fusarium outbreaks.
Conflict of Interest
The authors have declared no conflict of interest.
References
- Dellafiora L, Dall Asta C (2016) Masked mycotoxins: An emerging issue that makes renegotiable what is ordinary. Food Chem 213: 534-535.
- (2011) Food Standards Agency, Foodborne Disease Strategy. Scanning 4
- Berthiller F, Crews C, Dall Asta C, Saeger S De, Haesaert G, et al. (2013) Masked mycotoxins: A review. Mol Nutr Food Res 57(1): 165-186.
- Anfossi L, Giovannoli C, Baggiani C (2016) Mycotoxin detection. Curr Opin Biotechnol 37: 120-126.
- Freire L, Santana AS (2018) Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem Toxicol 111: 189-205.
- Sobrova P, Adam V, Vasatkova A, Beklova M, Zeman L (2010) Deoxynivalenol and its toxicity. Interdiscip Toxicol 3(3): 94-99.
- Abbas HK, Shier WT, Plasencia J, Weaver MA, Bellaloui N (2017) Mycotoxin contamination in corn smut (Ustilago maydis) galls in the field and in the commercial food products. Food Control 71: 57-63.
- Parry DW, Jenkınson P, McLeod L (1995) Fusarium ear blight (scab) in small grain cereals—a review. Plant Pathol 44: 207-238.
- Miedaner T, Cumagun CJR, Chakraborty S (2008) Population genetics of three important head blight pathogens Fusarium graminearum, pseudograminearum and F. culmorum. J Phytopathol 156: 129-139.
- N Matny O (2015) Fusarium Head Blight and Crown Rot on Wheat & Barley: Losses and Health Risks. Adv Plants Agric Res. 2(1).
- Wagacha JM, Muthomi JW (2007) Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Prot 26(7): 877-885.
- Berthiller F, Maragos CM, Dall Asta C (2016) Chapter 1: Introduction to masked mycotoxins. Issues Toxicol 2016-Janua: 1-13.
- Lee T, Han YK, Kim KH, Yun SH, Lee YW (2002) Tri13 and tri7 determine deoxynivalenol- and nivalenol-producing chemotypes of Gibberella zeae. Appl Environ Microbiol 68(5): 2148-2154.
- Chandler EA, Simpson DR, Thomsett MA, Nicholson P (2003) Development of PCR assays to Tri7 and Tri13 trichothecene biosynthetic genes, and characterisation of chemotypes of Fusarium graminearum, Fusarium culmorum and Fusarium cerealis. Physiol Mol Plant Pathol 62(6): 355-367.
- Jennings P, Coates ME, Walsh K, Turner JA, Nicholson P (2004) Determination of deoxynivalenol- and nivalenol-producing chemotypes of Fusarium graminearum isolated from wheat crops in England and Wales. Plant Pathol 53(5): 643-652.
- Ji L, Cao K, Hu T, Wang S (2007) Determination of deoxynivalenol and nivalenol chemotypes of Fusarium graminearum isolates from China by PCR assay. J Phytopathol 155(7-8): 505-512.
- Wang JH, Li HP, Qu B, Zhang JB, Huang T (2008) Development of a generic PCR detection of 3-acetyldeoxy-nivalenol-, 15-acetyldeoxynivalenol- and nivalenol-chemotypes of fusarium graminearum clade. Int J Mol Sci 9(12): 2495-2504.
- Yörük E, Yli Mattila T (2019) Class B-Trichothecene Profiles of Fusarium Species as Causal Agents of Head Blight. In: Satyanarayana T, Deshmukh SK, Deshpande M V (eds) Adv. Front. Mycol. Mycotechnology Basic Appl. Asp. Fungi. Springer Singapore, Singapore, pp. 347-376.
- Righetti L, Rolli E, Galaverna G, Suman M, Bruni R (2017) Plant organ cultures as masked mycotoxin biofactories: Deciphering the fate of zearalenone in micropropagated durum wheat roots and leaves. PLoS One 12(11): 1-17.
- Gareis M, Bauer J, Thiem J, Plank G, Grabley S (1990) Cleavage of Zearalenone-Glycoside, a “Masked” Mycotoxin, during Digestion in Swine. J Vet Med Ser B 37: 236-240.
- Berthiller F, Dall Asta C, Schuhmacher R, Lemmens M, Adam G (2005) Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J Agric Food Chem 53(9): 3421-3425.
- Rasmussen PH, Nielsen KF, Ghorbani F, Spliid NH, Nielsen GC (2012) Occurrence of different trichothecenes and deoxynivalenol- 3-β-D-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res 28(3): 181-190.
- Wu Q, Kuča K, Humpf HU, Klímová B, Cramer B (2017) Fate of deoxynivalenol and deoxynivalenol-3-glucoside during cereal-based thermal food processing: a review study. Mycotoxin Res 33(1): 79-91.
- Weidenbörner M (2011) Mycotoxins in Feedstuffs. Springer US
- Gardiner SA, Boddu J, Berthiller F, Hametner C, Stupar RM (2010) Transcriptome Analysis of the Barley–Deoxynivalenol Interaction: Evidence for a Role of Glutathione in Deoxynivalenol Detoxification. Mol Plant-Microbe Interact 23(7): 962-976.
- Poppenberger B, Berthiller F, Lucyshyn D, Sieberer T, Schuhmacher R (2003) Detoxification of the Fusarium Mycotoxin Deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J Biol Chem 278(48): 47905-47914.
- Rychlik M, Humpf HU, Marko D, Dänicke S, Mally A (2014) Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Res 30(4): 197-205.
- Kluger B, Bueschl C, Lemmens M, Michlmayr H, Malachova A (2015) Biotransformation of the mycotoxin deoxynivalenol in fusarium resistant and susceptible near isogenic wheat lines. PLoS One 10(3): e0119656.
- Warth B, Fruhmann P, Wiesenberger G, Kluger B, Sarkanj B (2015) Deoxynivalenol-sulfates: identification and quantification of novel conjugated (masked) mycotoxins in wheat. Anal Bioanal Chem 407(4): 1033-1039.
- Schneweis I, Meyer K, Engelhardt G, Bauer J (2002) Occurrence of zearalenone-4-beta-D-glucopyranoside in wheat. J Agric Food Chem 50: 1736-1738.
- Berthiller F, Sulyok M, Krska R, Schuhmacher R (2007) Chromatographic methods for the simultaneous determination of mycotoxins and their conjugates in cereals. Int J Food Microbiol 119(1-2): 33-37.
- De Boevre M, Di Mavungu JD, Maene P, Audenaert K, Deforce D (2012) Development and validation of an LC-MS/MS method for the simultaneous determination of deoxynivalenol, zearalenone, T-2-toxin and some masked metabolites in different cereals and cereal-derived food. Food Addit Contam Part A 29(5): 819-835.
- Kovalsky Paris MP, Schweiger W, Hametner C, Stückler R, Muehlbauer GJ (2014) Zearalenone-16-O-glucoside: a new masked mycotoxin. J Agric Food Chem 62(5): 1181-1189.
- Nathanail AV, Syvähuoko J, Malachová A, Jestoi M, Varga E (2015) Simultaneous determination of major type A and B trichothecenes, zearalenone and certain modified metabolites in Finnish cereal grains with a novel liquid chromatography-tandem mass spectrometric method. Anal Bioanal Chem 407(16): 4745-4755.
- Foroud NA, Eudes F (2009) Trichothecenes in cereal grains. Int J Mol Sci 11(11): 634.
- Bai X, Sun C, Xu J, Liu D, Han Y (2018) Detoxification of zearalenone from corn oil by adsorption of functionalized GO systems. Appl Surf Sci 430: 198-207.
- Bhat RV, Ramakrishna Y, Beedu SR, Munshi KL (1989) Outbreak of Trichothecene Mycotoxicosis Associated with Consumption of Mould-Damaged Wheat Products in Kashmir Valley, India. Lancet 1(8628): 35-37.
- Pestka JJ, Smolinski AT (2005) Deoxynivalenol: Toxicology and potential effects on humans. J Toxicol Environ Heal - Part B Crit Rev 8(1): 39-69.
- (2012) Mycotoxins and human health. IARC Sci Publ pp. 87-104.
- Shifrin VI, Anderson P (1999) Trichothecene mycotoxins trigger a ribotoxic stress response that activates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase and induces apoptosis. J Biol Chem 274(20): 13985-13992.
- Zhou HR, Jia Q, Pestka JJ (2005) Ribotoxic Stress Response to the Trichothecene Deoxynivalenol in the Macrophage Involves the Src Family Kinase Hck. Toxicol Sci 85(2): 916-926.
- Pestka JJ (2010) Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch Toxicol 84(9): 663-679.
- Lorenz N, Dänicke S, Edler L, Gottschalk C, Lassek E (2019) A critical evaluation of health risk assessment of modified mycotoxins with a special focus on zearalenone. Mycotoxin Res 35(1): 27-46.
- Liu J, Applegate T (2020) Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins (Basel) 12(6): 377.
- Weiden börner M (2011) Mycotoxins and Their Metabolites in Humans and Animals. Springer US
- Oliveira M, Vasconcelos V (2020) Occurrence of mycotoxins in fish feed and its effects: A review. Toxins (Basel) 12(3): 1-25.
- Broekaert N, Devreese M, Demeyere K, Berthiller F, Michlmayr H (2016) Comparative in vitro cytotoxicity of modified deoxynivalenol on porcine intestinal epithelial cells. Food Chem Toxicol 95: 103-109.
- Pierron A, Mimoun S, Murate LS, Loiseau N, Lippi Y (2016) Intestinal toxicity of the masked mycotoxin deoxynivalenol-3-β-d-glucoside. Arch Toxicol 90(8): 2037-2046.
- Yang Y, Yu S, Tan Y, Liu N, Wu A (2017) Individual and combined cytotoxic effects of co-occurring deoxynivalenol family mycotoxins on human gastric epithelial cells. Toxins (Basel) 9(3): 1-10.
- Videmann B, Mazallon M, Tep J, Lecoeur S (2008) Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem Toxicol 46(10): 3279-3286.
- Cirlini M, Barilli A, Galaverna G, Michlmayr H, Adam G (2016) Study on the uptake and deglycosylation of the masked forms of zearalenone in human intestinal Caco-2 cells. Food Chem Toxicol 98(Pt B) : 232-239.
- Nathanail AV, Jestoi M, Jonsson M, Peltonen K (2016) Chapter 6 In Vitro Assays to Estimate the Toxicological Effects of Masked Mycotoxins. In: Masked Mycotoxins Food Form. Occur. Toxicol. Relev The Royal Society of Chemistry pp. 97-136.
- Berthiller F, Krska R, Domig KJ, Kneifel W, Juge N (2011) Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol Lett 206(3): 264-267.
- De Nijs M, Van Den Top HJ, Portier L (2012) Digestibility and absorption of deoxynivalenol-3-ß-glucoside in in vitro models. World Mycotoxin J 5(3): 319-324.
- Dall Erta A, Cirlini M, Dall Asta M, Del Rio D, Galaverna G (2013) Masked mycotoxins are efficiently hydrolyzed by human colonic microbiota releasing their aglycones. Chem Res Toxicol 26(3): 305-312.
- Boevre M De, Graniczkowska K, Saeger S De (2015) Metabolism of modified mycotoxins studied through in vitro and in vivo models: An overview. Toxicol Lett 233(1): 24-28.
- Gratz SW (2017) Do plant-bound masked mycotoxins contribute to toxicity? Toxins (Basel) 9(3): 85.
- Zhang Z, Nie D, Fan K, Yang J, Guo W (2020) A systematic review of plant-conjugated masked mycotoxins: Occurrence, toxicology, and metabolism. Crit Rev Food Sci Nutr 60(9): 1523-1537.
- Yang S, Zhang H, Zhang J, Li Y, Jin Y (2018) Deglucosylation of zearalenone-14-glucoside in animals and human liver leads to underestimation of exposure to zearalenone in humans. Arch Toxicol 92(9): 2779-2791.
- Gratz SW, Duncan G, Richardson AJ (2013) The human fecal microbiota metabolizes deoxynivalenol and deoxynivalenol-3-glucoside and may be responsible for urinary deepoxy-deoxynivalenol. Appl Environ Microbiol 79: 1821-1825.
- Veršilovskis A, Geys J, Huybrechts B, Goossens E, De Saeger S (2012) Simultaneous determination of masked forms of deoxynivalenol and zearalenone after oral dosing in rats by LC-MS/MS. World Mycotoxin J 5(3): 303-318.
- Gratz SW, Currie V, Richardson AJ, Duncan G, Holtrop G (2018) Porcine small and large intestinal microbiota rapidly hydrolyze the masked mycotoxin deoxynivalenol-3- glucoside and release deoxynivalenol in spiked batch cultures in vitro. Appl Environ Microbiol 84(2): 1-9.
- Nagl V, Schwartz H, Krska R, Moll WD, Knasmüller S (2012) Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in rats. Toxicol Lett 213(3): 367-373.
- Nagl V, Woechtl B, Schwartz Zimmermann HE, Hennig Pauka I, Moll WD (2014) Metabolism of the masked mycotoxin deoxynivalenol-3-glucoside in pigs. Toxicol Lett 229(1): 190-197.
- Binder SB, Schwartz Zimmermann HE, Varga E, Bichl G, Michlmayr H (2017) Metabolism of zearalenone and its major modified forms in pigs. Toxins (Basel) 9(2): 1-15.
- Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B (2017) Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 15(7): 4851.
- Catteuw A, Devreese M, De Baere S, Antonissen G, Ivanova L (2020) Investigation of age-related differences in toxicokinetic processes of deoxynivalenol and deoxynivalenol-3-glucoside in weaned piglets. Arch Toxicol 94(2): 417-425.
- Panel E, Chain F (2014) Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA J 12(12): 3916.
- Guo H, Ji J, Wang J sheng, Sun X (2020) Deoxynivalenol: Masked forms, fate during food processing, and potential biological remedies. Compr Rev Food Sci Food Saf 19(2): 895-926.

 Mini Review
Mini Review