Abstract
We describe an outbreak of bacteremia due to carbapenem resistant Klebsiella pneumoniae carbapenemase-2 (KPC-2)- producing Klebsiella pneunoniae occurred in the Hematology Department of a tertiary care Hospital in Greece. From November 2016 through May 2019, 16 patients with hematologic malignancies, with prolonged history of hospitalization and prolonged administration of antibiotics, were colonized (n= 3), or infected (n= 13) by KPC-2-producing K.pneumoniae. Clinical diagnoses included pneumonia (50 % of cases), bacteremia (75 %) and urinary tract infection (12 %). Overall, 18 KPC-producing K.pneumoniae isolates, mainly from blood (94 %), urine (25%), respiratory (31%), gastrointestinal tract (31%) and catheter tip (6%) were identified. Most patients received a colistin-containing combination treatment. Crude mortality was 93,8% but attributable mortality was 68,8%. The emergence of KPC-producing K. pneumoniae is associated with significant morbidity and mortality among hematologic patients, and warrants focus on rational use of antibiotics, enhancement of infection control measures and implementation of antibiotic resistance surveillance.
Keywords: Bacteraemia; KPC-2-Producing K.Pneumoniae; Outbreak; Hematologic Disorders
Research Article
Carbapenems are frequently used for severe hospital infections caused by K. pneumoniae, especially when isolates produce extended-spectrum β-lactamases (ESBLs) or have chromosomal cephalosporinases. The last decade, several studies have documented the emergence of carbapenem resistant K. pneumoniae (CR-KP) worldwide and in Greece. Data from the Greek System for the Surveillance of Antimicrobial Resistance [1] show that among K. pneumoniae blood isolates, carbapenem resistance increased from <1% in 2001 to 42% in medical wards and to 72% in intensive care units (ICUs). Carbapenem resistance is mainly due to the production of carbapenemases which is considered to be the most important molecular mechanism. Carbapenemases belong to four molecular families but Classes A and B and D are of greater clinical importance. Classes A and B are distinguished by the hydrolytic mechanism at the active site. The carbapenemases of Class A utilize serine at their active sites, with KPC being the main representative. The carbapenemases of Class B contain at least one zinc atom at the active site, establishing them as metalloenzymes, with VIM and IMP representing the most prevalent enzymes [2]. In 2001, the first KPC-producing K. pneumoniae isolate was reported in North Carolina [3] and then several reports documented the emergence of Enterobacteriaceae strains from various species producing b-lactamase KPC-2 and KPC-3 variants in the eastern USA and recently disseminated in other countries worldwide, such as France, Colombia, Israel and China [4-6]. There were two publications, one in late 2007 and the other in early 2008, reporting infections due to KPC-producing K. pneumoniae in two patients, one in Sweden and the other in France. Both patients had originally been hospitalized in Crete, Greece [7,8]. The same time period two other outbreaks of infections due to K. pneumoniae were identified in two Greek hospitals [9,10]. A surveillance study organized from February through December 2008 at 21 hospitals in Greece identified the presence of KPC-2–producing K. pneumoniae at 18 hospitals in Athens, Crete, and Thessaloniki. Among the 171 isolates studied, 97.1% belonged to the same pulse type, which was found at 17 hospitals, suggesting a nationwide dissemination of a hyperepidemic clone [11,12]. The aim of the present study was to describe the epidemiologic, microbiologic and clinical characteristics of the emergence of KPC-2 producing K. pneumoniae outbreak at a General Hospital, in Athens.
Material and Methods
Setting
We conducted a retrospective study of KPC-2 producing K. preunoniae bacteremias occurring from November 2016 through May 2019, in the 42-bed adult Hematology department of the General Hospital of Athens “G. Gennimatas”. This department includes a 15-bed day care Center, a 25-bed ward and a two- bed isolation unit. All rooms have their own toilet and washroom. During neutropenia, patients are cared for using strict contact and respiratory isolation procedures.
Bacteriological Studies
The identification and the initial susceptibility testing of K. pneumoniae strains isolated from clinical samples were performed by Kirby-Bauer method. MICs of imipenem, meropenem, ertapenem, tigecycline and colistin were determined by Vitek II automated system (bioMerieux, France) and by E-test method (AB Biodisk, bioMerieux) according to CLSI guidelines and interpretative criteria [13]. Suspect strains from colonization and infection were forwarded to the National School of Public Health in Athens for further identification. The presence of the blaKPC gene was confirmed by PCR using forward and reverse primers proposed by Queenan and Bush [2] and subsequent sequencing on both strands of the PCR products. Molecular typing was performed by pulsed field gel electrophoresis (PFGE) of Xbal-restricted genomic DNA [14]. Restriction fragments were separated through a 1% agarose using a contour-clamber homogeneous electric field DRIII apparatus (BioRad, Milano, Italy). Gel Compar II was used for classification of the isolates into PFGE types [11].
Infection Control Policies
Oropharyngeal and rectal swab samples were collected upon admission and weekly in all admitted patients in the Hematology ward. During the investigation of the outbreak, environmental samples were collected from the furniture as well as from tap and toilet water of the rooms. All medical and nurse staff used single-use gloves and facial masks and alcohol-based disinfectant. Each patient used facial mask and patient-allocated equipment and strict visiting rules were implemented for both patients and their visitors (limited visiting hours, only one visitor per patient, use of single-use gowns and facial masks).
Antimicrobial Management
Systemic antimicrobial prophylaxis is not routinely administered in our Center. The first line empirical combination, in febrile neutropenia, includes the combination of piperacillin-tazobactam or cefepime plus amikacin. Imipenem is used in second line regimen. Colistin is not included in standard guidelines and is used, where is imposed, at a dose of 4,5 million units as twice daily infusions.
Data Collection and Definitions
We retrospectively reviewed the medical records of patients with KPC-producing K. pneumoniae infections and collected demographic, clinical, and microbiological data. Clinical infections versus hospitalization and the outcome of infected patients were defined. Hospitalization for more than 2 days prior the isolation of KPC-producing K. pneumoniae was suggestive of acquisition in the Hematology ward. Patients who died during the 14-day period were considered clinical failures. Survived patients were considered to have microbiologic failure if subsequent cultures remained positive 3 days or more after the introduction of appropriate treatment. Nosocomial infections were defined by standard CDC definitions [15]. This study was approved by the Ethical Review Board of the “G. Gennimatas” General Hospital, Athens, Greece. Informed consent for the study was obtained from all patients.
Result
KPC-2 producing K.pneumoniae strains were isolated from 16 patients and 18 episodes of infections were identified (two patients developed KPC infection twice). The patients’ characteristics are presented in Table 1. Acute myeloid leukemia was the underlying disease in 10 patients, non-Hodgkin lymphoma in two and acute lymphoblastic leukemia, chronic lymphoblastic leukemia, aplastic anemia and multiple myeloma, one each. Seven of them were males and nine were females and their median age was 70 years (range: 25-83). The median duration between admission and the isolation of KPC-2 strains was 24 days. Twelve patients were neutropenic during the bacteremias. Colonization with identical strains were present either before (upper respiratory and rectal in two patients, upper respiratory in one) or after the bacreremias (rectal colonization in three patients, upper respiratory tract in one). In four cases, the same isolated strain was concurrently identified in blood and other biological fluids. In three patients the strain was isolated from urine, pus, skin lesions and central venous catheter entry points. In another patient the isolated strain was later identified, in pus, sputum and in bronchoalveolar lavage.
Table 1: Clinical characteristics of 16 patients colonized or infected with KPC-producing K. Pneumoniae.
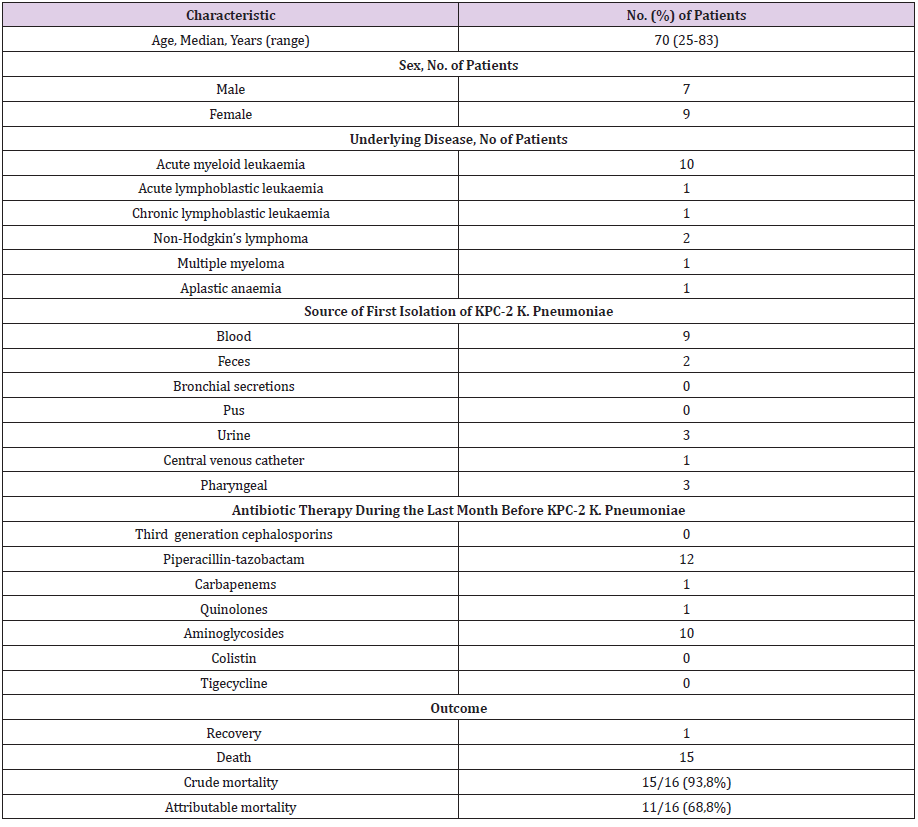
Colonization or bacteremia from multiple pathogens, including multidrug resistant Pseudomonas aeruginosa, K. pneumoniae (ESBL), vancomycin resistant Enterococcus and various fungi were concurrently identified in 12 patients with bacteremia from K. pneumoniae. All of the cases had either multiple or prolonged hospitalizations and all of them had received antibiotics for prophylaxis or treatment prior to the isolation of the pathogen. The phenotype of antibiotic resistance was common in all of the strains isolated nad was characterized by resistance to carbapenems (imipenem, meropenem, ertapenem) and quinolones (MIC≥ 4μg/ml) and by sensitivity to colistin, gentamicin, tetracycline and tigecycline. The molecular investigation identified them as KPC-2 producers, belonging to the same clone (palsotype A), (Figure 1).
Figure 1: Molecular typing of representative K. pneumoniae clinical isolates. Line 1 Lambda Ladder PFG Marker; lines 2-7 KPC-producing K. pneumoniae.
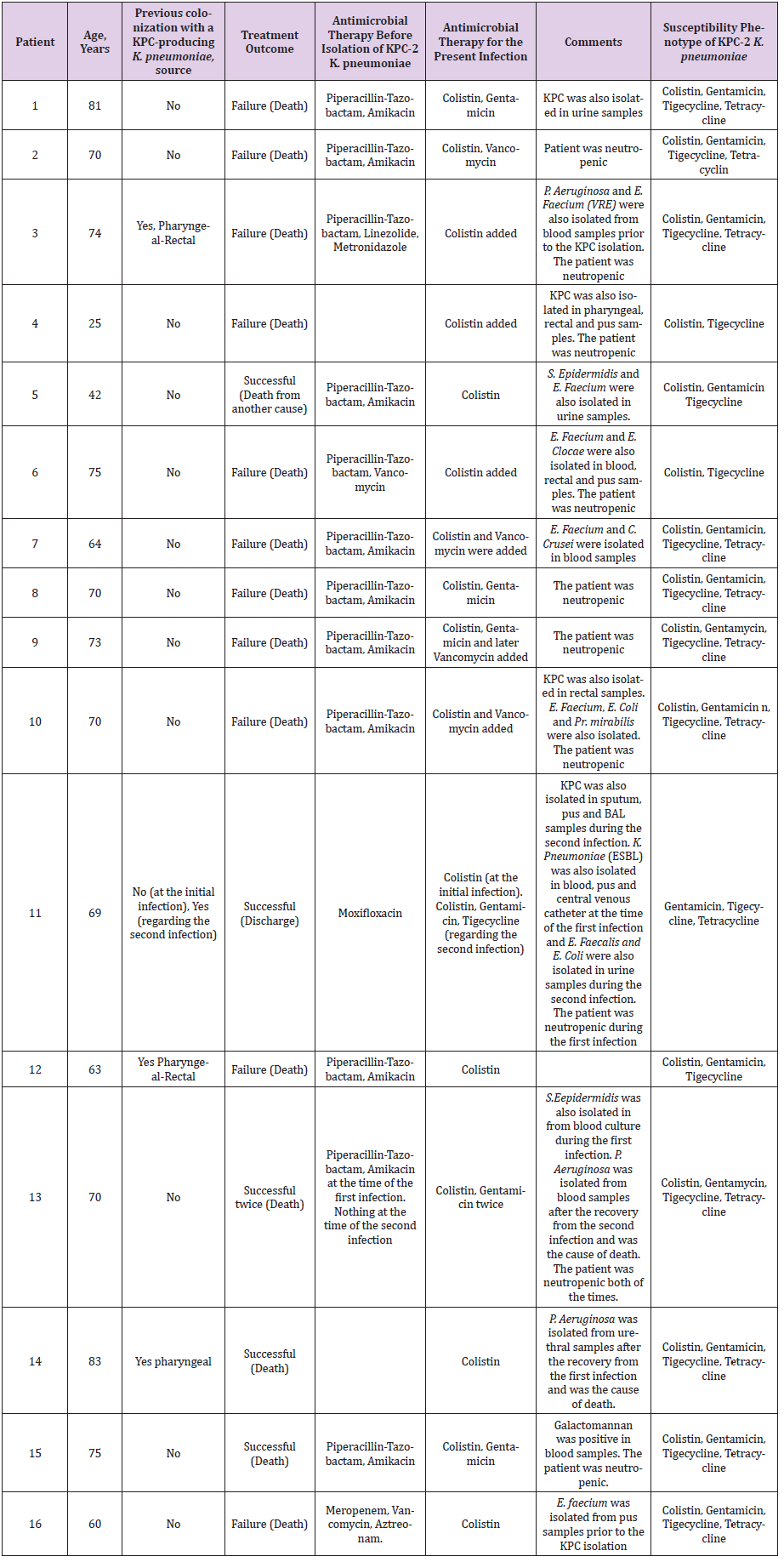
All of the cases were treated with a combination of antibiotics (piperacillin / tazobactam+amikacin) as first line treatment during the period of the first positive blood culture. After the isolation of the pathogen all of them were treated with an antibiotic regimen containing colistin or gentamicin and tigecycline. Although the period between the onset of symptoms and the administration of an active antibiotic regimen was short, 13 of the patients had already developed sepsis and were supported with dopamine and/or norepinephrin. Among the 16 patients, infection due to KPC- producing K. pneumoniae was considered to have contributed to death in 11/16 (68,8 %). One patient died of brain hemorrhage (not attributed to his infection), three died of infections caused by other bacteria, after having survived the KPC infection, and three survived. The clinical characteristics and outcomes of the 16 patients are shown in Supplemental Table 2.
Table 2: Demographic, clinical characteristics and outcome of 16 patients with clinical infection with KPC-producing K. Pneumoniae.
Discussion
The emergence of multidrug-resistant Gram-negative bacteria causing nosocomial infections is growing worldwide and has become a great challenge especially for clinicians and the Public Health System. KPC-producing bacteria are frequently misclassified as carbapenem-susceptible by routine susceptibility testing and should be suspected with ertapenem resistance [16]. We describe an outbreak of KPC-2-producing K. pneumoniae infection at the Hematology Department of a Greek Hospital highlighting the high mortality in the cohort of our patients. Prolonged hospitalization, ICU admission, mechanical ventilation, solid organ and stem cell transplantation, poor functional status and prolonged antimicrobial exposure to cephalosporins, fluoroquinolones, carbapenems and glycopeptides have been implicated as risk factors for acquisition of KPC-2-producing K. pneumoniae [17]. In our study, all infected patients with the KPC-producing strain had previously been treated with extended spectrum cephalosporins which may have contributed to the selection of KPC-2-producing K. pneumoniae strains in our setting, along with other risk factors as prolonged hospitalization and immunosuppression.
The outbreak strain corresponds to the predominant KPC variant (KPC-2) which has been recently published [18]. Currently, not only the KPC-2 enzyme but also the VIM-type is spread in Greece [19,20]. Recent epidemiology studies have shown that the outbreak of MBL-producing K. pneumoniae infection was polyclonal, while that of KPC-2 producing K. pneumoniae infection was monoclonal [11,12]. The epidemiological profile of the present outbreak was monoclonal similar to that described in US hospitals [21,22] and different from the polyclonal profile of the outbreak observed in Israel [6]. In our study, the monoclonal type of the outbreak and failure to isolate the implicated pathogen in the environmental stuff, implicates the poor adherence to hand hygiene among health-care personnel and patient-to-patient spread of the pathogen as the main mechanism of transmission of the KPC strain, as was also reported in previous studies [12].
Based on the susceptibility profile of the isolates, our patients were treated with combinations containing colistin, gentamicin and tigecycline. The optimal treatment for infections caused by KPC-producing isolates is still unknown. Agents shown to have in vitro activity against isolates harbouring KPCs include tigecycline, tetracyclines, polymyxins and the aminoglycosides. Tigecycline, a broad-spectrum, bacteriostatic minocycline derivative, is not considered adequate monotherapy for CR-KP infections, especially for nosocomial pneumonia and bloodstream infections [23,24]. Colistin, an antimicrobial peptide with activity against Gram-negative pathogens, known since 1950, is used for the treatment of carbapenem-resistant organisms. It is a concentration-dependent bactericidal antibiotic against CR-KP and other carbapenem resistant strains. Higher dose colistin regimens are relatively effective with acceptable and reversible nephrotoxicity in the treatment of life-threatening infections, provided doses are adjusted in accordance with renal function [24]. Among aminoglycosides, gentamicin is an active agent against some CR-KP strains and can be an important component of combination regimens in bloodstream or urinary tract infections. Although isolates are carbapenem-resistant in vitro, recent studies reported that carbapenem-containing combination regimens had significantly lower mortality rate compared with patients who received non carbapenem-containing regimens (12 vs 41%; p=0,006), especially in cases where the MIC of the infecting isolate was <4mg/l [25].
In our study, the crude and attributable mortality rates (93,8% and 68,8%, respectively) associated with CR-KP bacteremia were striking, much higher than those reported in previous studies. High crude and attributable mortality rates (71,9% and 50%, respectively), but still lower than ours, have been recently reported in Israel [26]. Mortality rates ranging from 25 to 47% have been reported by others with fatal outcome in bacteremia cases [21,27,28]. Our study included only hematological patients, most of whom were elderly with previous long hospitalizations after multiple cycles of chemotherapy and neutropenic during the isolation of the CR-KP strain, having different and significant comorbidities. Of note, all patients had blood stream infection (previous studies were not focused on bacteremia) and 70,5% were in septic shock. Neutropenic febrile patients who present in shock have extremely poor outcomes irrespective of type of initial antibiotic therapy with similar high mortality rates of 82% [27,28]. Concomitant colonization or infections with other pathogens such as multidrug resistant P. Aeruginosa, VRE and fungi (in 61% of patients) might have aggravated the overall mortality rate. The strict implementation of the infection control measures, especially health-care personnel adherence to recommendations for gown and glove use at the hospital, appropriate hand hygiene (hand washing or using a waterless alcohol-based hand rub before and after patient contact), patient cohorting, pharyngeal and perirectal surveillance cultures in newly admitted and already hospitalized patients, finally resulted to control the outbreak [29,30].
Conclusion
In summary, we described the emergence of KPC-2 producing K.pneumoniae in a Hematology Unit, which is still a major problem between immunocompromised patients, with striking mortality rates in hospitalized hematology patients. This condition underscores the urgent need for institutions to develop strategies to limit and control carbapenem-resistant outbreaks which warrants a good collaboration between clinicians, pharmacologists, microbiologists and infection control professionals.
Conflict of Interest
Nil.

 Research Article
Research Article