ABSTRACT
Uterine myomas (leiomyomas or fibroids) are the most common benign tumors in women and have a significant impact on quality of life. The lack of highly effective noninvasive medical treatments positions a surgical technique as a prevalent therapeutic option. Recently, a new class of drugs, the selective progesterone receptor modulator (SPRM), has become available for the conservative management of myomas as a candidate of alternative medical managements.
Introduction
Uterine myomas are the most common benign tumor in women at reproductive age, with a prevalence of 70% to 80% in women by age 50 years [1-4]. Although many myomas are asymptomatic, 20% to 50% of patients develop a variety of symptoms over time, including abdominal bleeding and hypermenorrhea, subsequent anemia, pelvic pain, dysmenorrhea, infertility, and obstetric complications. The lack of highly effective non-invasive medical treatments positions a surgical technique as a common therapeutic option [5-8]. Recently, a new class of drugs, the selective progesterone receptor modulators (SPRM), has become available for the conservative management of myomas [9-13]. Recent excellent reports have documented the surgical and traditional non-surgical treatment options for uterine myomas [5-8]. The aim of this document is to review the known SPRM effects to understand as a potential suppressor of uterine myoma growth.
Progesterone Role in Uterine Myoma Growth
It has been discovered the critical role of progesterone pathways in the pathophysiology of uterine myomas [14-16]. Myoma express higher levels of both types of progesterone receptor (PR) isoforms; PR-A and PR-B [17]. Therefore, progesterone and PR play potential targets for modulating the growth of uterine myoma. SPRMs compete at the PR binding site in a tissue-specific and dosedependent manners. Covering by the SPRMs of the PR causes a mix of agonistic and antagonistic effects. The relative strength of the agonistic and antagonistic action may be balanced by the ratios of PR-A to PR-B in the particular tissue and the SPRM’s specific affinity for each receptor isoform. By acting on PRs throughout the reproductive system, SPRMs lead to several effects that assist in myoma node shrinkage and bleeding control. These include direct antiproliferative and proapoptotic actions against myoma cells, endometrial changes that ameliorate bleeding, and decline of the luteinizing hormone surge form anterior pituitary leading to subsequent anovulation and amenorrhea. Several previous studies including ours [18] compared an oral progestogen dienogest, with gonadotropin-releasing hormone agonist (GnRHa), and showed a significant decrease in myoma volume with both treatments (50% and 60%, respectively (Figure 1a).
Treatment of uterine myoma with progestogens may be effective in certain cases. The early SPRM, mifepristone has been studied in randomized trials with placebo and GnRHa. Mifepristone is effective in controlling symptoms and improving patient QOL in uterine myomas [19]. Although mifepristone is used in a limited region to treat uterine myoma, any jurisdiction does not allow to use for formal indication.
SPRMs and Uterine Myoma Treatment
SPRMs, such as mifepristone telapristone acetate, asoprisnil, vilaprisan, and ulipristal acetate [17-23] reduce the size of uterine myoma (Figure 1b), decrease menstrual blood loss, achieve amenorrhea, and subsequently improve myoma-related symptoms. In recent years, ulipristal acetate, which has been approved as an emergency contraceptive in the USA, has been the focus of clinical research [24-28]. Ulipristal acetate has been shown to reduce myoma volume, and induce amenorrhea in most of treated women, and is currently approved for clinical use primarily in Europe and Canada. Ulipristal acetate is a synthetic steroid derived from 19-norprogesterone, an SPRM that binds to PR-A and PR-B with high affinity [29-31]. The binding and antagonism potency of ulipristal acetate to other steroid receptor is significantly lower when compared to mifepristone and tissue-selective, preferential to the uterine corpus, cervix, ovaries, and anterior pituitary/ hypothalamus [29-31]. Repeated courses of ulipristal acetate are safe and effective in maintaining long-term control of myoma symptoms, with therapeutic effects lasting at least 3 months after treatment discontinuation [24-28]. In the PGL4001 (ulipristal acetate) efficacy assessment in reduction of symptoms due to uterine leiomyomata (PEARL) trials [25-27, 32-34], at least 70% of patients end up the primary endpoint of amenorrhea throughout each treatment process. The median time to amenorrhea takes 4 to 6 days.
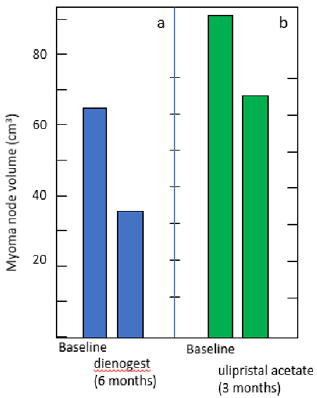
Figure 1: Myoma node shrinkage in patients treated with dienogest (a) and ulipristal acetate (b).
(a) Premenopausal patients with endometriosis, who were associated with coexistent myoma node (> 30 cm3), received dienogest (2mg daily) for 6 months. The total volume of myoma declined to 59.7 % ± 5.5 %), according to Ichigo, et al. [18].
(b) The patients with myoma nodes (> 30cm3) were treated with ulipristal acetate (5mg daily) for 3 months. The mean myoma shrinkage amounted 19.62 %. according to Szydłowska, et al [12].
Bleeding decline occurred in approximately 70% of subjects, and the intensity of menstrual bleeding decreased with each successive course of treatment. Myoma node volume also significantly decreased with each course of treatment, and this decrease was maintained through the 3-month follow-up after treatment. From a long-term safety perspective, overall adverse events were similar in rate and severity to those seen the shorter-term trials; the most common were headaches and hot flashes. The number of nodes and the age of patients do not interfere with ulipristal acetate efficacy. To our knowledge, researchers seem to have paid little attention to coexistence of endometriosis or adenomyosis. Uterine myomas and endometriosis/adenomyosis have many common features and known to have frequent rate of coexistence of these conditions [35]. These are estrogen-dependent conditions that can often be source of pelvic pain and menstrual abnormalities. We reported successful management of a series of patients associated with endometriosis by use of a perogestine dienogest [18]. Clearly, these proposals will need additional intense clinical research on patients with adenomyosis-complicated uterine myoma who wish to avoid surgical intervention.
Conclusion
Ulipristal acetate may be a candidate for an efficient method in preoperative preparation women with symptomatic uterine myomas and is also available for safe non-surgical fertility preservation. This medical myomectomy may also support the concept of conversion of primary and secondary prevention of uterine myomas of pre-symptomatic and early symptomatic women. This area is well advanced and provides a powerful uterine myoma risk assessment tool to identify women who are either racially or genetically predisposed the development of future uterine myomas. Further studied are urgently needed to delineate the proper position of ulipristal acetate as well as other SPRMs in the anti-uterine myoma management strategies.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Stewart EA (2015) Clinical practice. Uterine fibroids. N Engl J Med 372: 1646-1655.
- McWilliams MM, Chennathukuzhi VM (2017) Recent advances in uterine fibroid etiology. Semin Reprod Med 35: 181-189.
- Whynott RM, Vaught KCC, Segars JH (2017) The effect of uterine fibroids on infertility: A systematic review. Semin Reprod Med 35: 523-532.
- Havryliuk Y, Setton R, Carlow JJ, Shaktman BD (2017) Symptomatic fibroid management: Systematic review of the literature. JSLS 21: e2017.00041.
- Donnez J, Dolmans MM (2016) Uterine fibroid management: From the present to the future. Hum Reprod Update 22: 665-686.
- De La Cruz MS, Buchanan EM (2017) Uterine fibroids: Diagnosis and treatment. Am Fam Physician. 95: 100-107.
- Sohn GS, Cho S, Kim YM, Cho CH, Kim MR, et al. (2018) Current medical treatment of uterine fibroids. Obstet Gynecol Sci 61(2): 192-201.
- Singh SS, Belland L, Leyland N, von Riedemann S, Murji A (2018) The past, present, and future of selective progesterone receptor modulators in the management of uterine fibroids. Am J Obstet Gynecol 218: 563-572.e1.
- Murji A, Whitaker L, Chow TL, Sobel ML (2017) Selective progesterone receptor modulators (SPRMs) for uterine fibroids. Cochrane Database Syst Rev 4: CD010770.
- Stewart EA, Diamond MP, Williams ARW, Carr BR, Myers ER, et al. (2019) Safety and efficacy of the selective progesterone receptor modulator asoprisnil for heavy menstrual bleeding with uterine fibroids: Pooled analysis of two 12-month, placebo-controlled, randomized trials. Hum Reprod. 34: 623-634.
- Ali M, Al Hendy A (2017) Selective progesterone receptor modulators for fertility preservation in women with symptomatic uterine fibroids. Biol Reprod 97: 337-352.
- Szydłowska I, Marciniak A, Nawrocka Rutkowska J, Rył A, Starczewski A (2020) Predictive factors of response to selective progesterone receptor modulator (ulipristal acetate) in the pharmacological treatment of uterine fibroids. Int J Environ Res Public Health 17: 798.
- Rabe T, Saenger N, Ebert AD, Roemer T, Tinneberg HR, et al. (2018) Selective progesterone receptor modulators for the medical treatment of uterine fibroids with a focus on ulipristal acetate. Biomed Res Int 2018: 1374821.
- Kim JJ, Sefton EC (2012) The role of progesterone signaling in the pathogenesis of uterine leiomyoma. Mol Cell Endocrinol 358: 223-231.
- Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, (2010) Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology 151(6): 2433-2442.
- Tsigkou A, Reis FM, Lee MH, Jiang B, Tosti C, et al. (2015) Increased progesterone receptor expression in uterine leiomyoma: correlation with age, number of leiomyomas, and clinical symptoms. Fertil Steril 104: 170-175.
- Donnez J, Dolmans MM (2016) Uterine fibroid management: from the present to the future. Hum Reprod Update 22: 665-686.
- Ichigo S, Takagi H, Matsunami K, Suzuki N, Imai A (2011) Beneficial effects of dienogest on uterine myoma volume: A retrospective controlled study comparing with gonadotropin-releasing hormone agonist. Arch Gynecol Obstet 284: 667-670.
- Tristan M, Orozco LJ, Steed A, Ramírez Morera A, Stone P (2012) Mifepristone for uterine fibroids. Cochrane Database Syst Rev 15: CD007687.
- Feng C, Meldrum S, Fiscella K (2010) Improved quality of life is partly explained by fewer symptoms after treatment of fibroids with mifepristone. Int J Gynaecol Obstet. 109: 121-124.
- Kulshrestha V, Kriplani A, Agarwal N, Sareen N, Garg P, et al. (2013) Low dose mifepristone in medical management of uterine leiomyoma - an experience from a tertiary care hospital from north India. Indian J Med Res. 137: 1154-1162.
- Murphy AA, Morales AJ, Kettel LM, Yen SS (1995) Regression of uterine leiomyomata to the antiprogesterone RU486: Dose-response effect. Fertil Steril 64(1): 187-190.
- Reinsch RC, Murphy AA, Morales AJ, Yen SS (1994) The effects of RU 486 and leuprolide acetate on uterine artery blood flow in the fibroid uterus: A prospective, randomized study. Am J Obstet Gynecol 170: 1623-1627.
- Luyckx M, Squifflet JL, Jadoul P, Votino R, Dolmans MM, et al. (2014) First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil Steril 102(5): 1404-1409.
- Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, Zakharenko NF, et al. (2012) Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med 366(5): 409-420.
- Donnez J, Tomaszewski J, Vázquez F, Bouchard P, Lemieszczuk B, et al. (2012) Ulipristal acetate versus leuprolide acetate for uterine fibroid. PEARL II Study Group. N Engl J Med 366(5): 421-432.
- Donnez J, Vázquez F, Tomaszewski J, Nouri K, Bouchard P, Fet al. (2014) Long-term treatment of uterine fibroids with ulipristal acetate. PEARL III and PEARL III Extension Study Group. Fertil Steril 101(6): 1565-1573.e1-18.
- Fauser BC, Donnez J, Bouchard P, Barlow DH, Vázquez F, et al. (2017) Safety after extended repeated use of ulipristal acetate for uterine fibroids. PLoS One 12(3): e0173523.
- Biglia N, Carinelli S, Maiorana A, D'Alonzo M, Lo Monte G, et al. (2014) Ulipristal acetate: A novel pharmacological approach for the treatment of uterine fibroids. Drug Des Devel Ther 8: 285-292.
- Courtoy GE, Donnez J, Marbaix E, Dolmans MM (2015) In vivo mechanisms of uterine myoma volume reduction with ulipristal acetate treatment. Fertil Steril 104(2): 426-434.e1.
- Nieman LK, Blocker W, Nansel T, Mahoney S, Reynolds J, et al. (2011) Efficacy and tolerability of CDB-2914 treatment for symptomatic uterine fibroids: Arandomized, double-blind, placebo-controlled, phase IIb study. Fertil Steril 95(2): 767-772.e1-2.
- Powell M, Dutta D (2016) Esmya and the PEARL studies: A review. Womens Health (Lond) 12(6): 544-548.
- Donnez J, Hudecek R, Donnez O, Matule D, Arhendt HJ, et al. (2015) Efficacy and safety of repeated use of ulipristal acetate in uterine fibroids. Fertil Steril 103(2): 519-527.
- Donnez J, Donnez O, Matule D, Ahrendt HJ, Hudecek R, et al. (2016) Long-term medical management of uterine fibroids with ulipristal acetate. Fertil Steril 105: 165-173.e4.
- Huang JQ, Lathi RB, Lemyre M, Rodriguez HE, Nezhat CH, et al. (2010) Coexistence of endometriosis in women with symptomatic leiomyomas. Fertil Steril. 94: 720-723.

 Mini Review
Mini Review