Abstract
Introduction the Perennial Pandemic: It is being increasingly realized that the Covid-19 may have become the new reality associated with human existence world over and the mankind may have to live with it for years or even decades. Further, the grievous nature of the disease is evolving further with the genomic changes in the virus in form of mutations and evolution of variants, with enhanced infectivity and probably virulence. There are serious challenges posed by the SARS-CoV-2 virus and COVID-19 as the disease.
Acute and Chronic Phases of Covid-19: Further, it is becoming clear that apart from the advanced age and pre-existing conditions, such as diabetes, cardiovascular, pulmonary, and renal diseases, certain constituent factors render some patients more vulnerable to more severe forms of the disease. These factors influence the COVID-19 manifestations, its course, and later the convalescence period as well as the newly defined ‘long Covid’ phase. The substantial continuing morbidity after resolution of the infection indicates persisting multisystem effects of ‘long Covid’. Lung Damage Associated with Covid-19: COVID-19 is primarily a respiratory disease presenting with a broad spectrum of respiratory tract involvement ranging from mild upper airway affliction to progressive life-threatening viral pneumonia. It affects the respiratory system in various ways across the spectrum of disease severity, depending on age, immune status, and comorbidities. The symptoms may be mild, such as cough, shortness of breath and fever, to severe and critical disease, including respiratory failure, shock, and multi-organ failure.
Implications for the Post-Covid Care: Depending on the severity of respiratory inflammation and damage, as well as associated comorbidities, duration of injury and genetics, the progressive fibrosis leads to compression of lung tissue and damage to pulmonary microvasculature. The COVID-19 patients with moderate/severe symptoms are likely to have a significant degree of long-term reduction in lung function. Depending on the severity of the disease, extensive and long-lasting damage to the lungs can occur, which may persist well after the resolution of infection.
Managing the Long Covid’s Challenges: Given the global scale of the pandemic, the healthcare needs for patients with sequelae of COVID-19, especially in those with lung affliction are bound to increase in the near future. The challenge can be tackled by harnessing the existing healthcare infrastructure, development of scalable healthcare models and integration across disciplines with a combination of pharmacological and non-pharmacological modalities. Following clinical and investigational assessment, the therapeutic strategy is likely to depend on the clinical manifestations, extent of damage in lungs and other organs, and associated complications.
Keywords: Long Covid; Long Haulers; Ground Glass Opacities; Covid Pneumonia; ARDS; Pulmonary Fibrosis; Covid Pulmonary Clinics; Heart Failure; Pulmonary Care Continuum; Holistic Care
Introduction: The Evolving Pandemic
The SARS-CoV-2 Virus and COVID-19
Following infection, the SARS-CoV-2 virus becomes an intracellular entity. The intracellular replication, ensuing cellular damage, and involvement of various cells in respiratory system, immune system, and other organs, propels the clinical course of the disease. While we continue to explore the agent factors, disease transmission dynamics, pathogenesis and clinical spectrum of the disease, and therapeutic modalities, the grievous nature of the disease is evolving further with the genomic changes in the virus and pathophysiological alterations and clinical manifestations of the disease. Further, it may not be possible to eradicate the intracellular virions, which may persist and continue to carry on the inflammatory process leading to the ongoing organ damage and manifestations of ‘long Covid’. The future course of the disease, of course, is said to depend on various known and unknown factors, many of them may not be modifiable. There are serious challenges posed by SARS-CoV-2 virus and COVID-19 as the disease, that are further increased by mutations and evolution of variants with enhanced infectivity and probably virulence. The significance of viral mutations needs to be explored in this context, which may help in diagnostic workup, designing therapeutics to combat the disease and developing effective vaccines for its prophylaxis. In addition, the avenues for improving the immune response to the infection and following vaccine inoculation, and the immunity in general are to be explored and harnessed. It is being increasingly realised that the COVID-19 may have become the new reality associated with human existence world over and the mankind may have to live with it for years or even decades [1].
Clinical Spectrum of Acute Covid-19 Illness
COVID-19 is primarily a respiratory disease presenting with a broad spectrum of respiratory tract involvement ranging from mild upper airway affliction to progressive life-threatening viral pneumonia. Coming into contact with the mucous membranes lining nose, mouth, and occasionally eyes, the SARS-CoV-2 virus enters the host cells, multiplies intracellularly and the released virions infect other cells. As it attacks the cells, it travels down the airways, from upper to lower respiratory tracts, finally infecting the alveoli and manifesting as pneumonia. In general, a significant number of those infected with the SARS-CoV-2 virus are asymptomatic, and as documented in a recent systematic review, at least one-third of SARS-CoV-2 infections in people who are exposed remain asymptomatic [2]. The study included serologic surveys from more than 365,000 people in England and more than 61,000 in Spain. When analysed, a similar proportion of asymptomatic cases 32.4% in England and 33% in Spain was noted. On being exposed to the SARS-CoV-2 virus, not all infected patients develop the disease. For those who develop the disease, there is a large variation in disease severity, one component of which may be due to the genetic variability in the response to the virus [3]. The individual vulnerability to the infection, response following the SARS-CoV-2 exposure, and the clinical spectrum of COVID-19 are greatly variable.
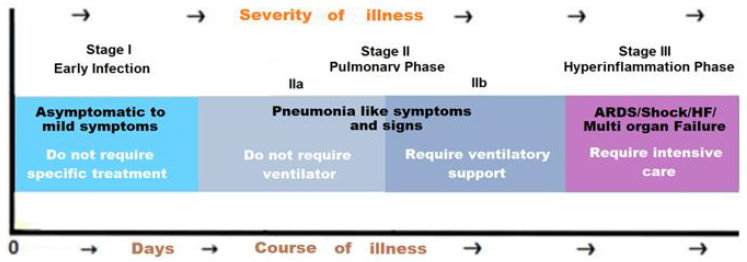
In general, older adults and people who have concurrent health conditions like heart disease, cancer, obesity, and diabetes are prone to develop serious manifestations. The clinical manifestations of COVID-19 illness have been grouped into Stages I to III, based on the need for hospitalization, need for oxygen supplementation, progression to respiratory failure, and other parameters of the disease severity (Figure 1). Presently, considering the delayed post-illness convalescence and persisting clinical manifestations, the resolution phase can be added to this, as Stage IV. COVID-19 affects the respiratory system in various ways across the spectrum of disease severity, depending on age, immune status, and comorbidities. The patients with underlying lung disease such as asthma, chronic obstructive pulmonary disease (COPD), interstitial lung disease, etc. can suffer with worsening of respiratory conditions. The symptoms may be mild, such as cough, shortness of breath and fever, to severe and critical disease, including respiratory failure, shock, and multi-organ failure (MOF). In general, most people who develop clinical COVID-19, manifest mild to moderate symptoms [4]. They may have a dry cough or sore throat, whereas a minority of them goes on to develop pneumonia, with ground-glass opacities on a chest CT scan. Further, about 14% of COVID-19 cases have severe infection affecting both lungs.
The air sacs become filled with mucus and proteinous fluid and infiltrated by immune calls leading to shortness of breath and dyspnoea and hypoxia. On the serious side of the clinical spectrum, about 5% of all COVID-19 cases suffer with critical disease with extensive damage to the airways and the alveoli, developing into severe pneumonia or acute respiratory distress syndrome (ARDS). Further, about 20-30% of critically ill patients can develop clots in the lungs, heart, brain, and legs. There may occur disseminated intravascular coagulation (DIC).
The Phases of SARS-CoV-2 Lung Infection
When the SARS-CoV-2 infection starts spreading into the respiratory tract and lungs, it triggers various symptoms and complications, and may be associated with constant coughing often without phlegm, and pain in chest. The pathophysiological processes underlying COVID-19 illness may or may not show clinical features of earlier or later phases.
Phase 1: Cell Invasion and Viral Replication: The SARS-CoV-2 virus gains entry via ACE2 receptors, which are present on goblet (secretory) cells and on ciliated (hairy) cells in the nose, and through ACE2 receptors in the mouth and tongue.
Phase 2: Viral Replication and Immune Response: As part of the defensive immune response, the lymphocytes begin to produce the IgM-type antibodies at first and later the longer-term specific neutralizing antibodies (the IgG type). In a German study, about 50% of the participants showed circulating IgM or IgG antibodies by day 7, and by day 14 all of them had developed antibodies [5]. Here, it is noteworthy that the antibodies titre may not predict the clinical course of the disease [6].
Phase 3: Lung Inflammation and Pneumonia: Approximately 13.8% of people with COVID-19 suffer with dyspnoea and severe disease and require hospitalization. Out of these, three-fourth patients may have evidence of bilateral pneumonia [7]. The pneumonia in COVID-19 manifests as consolidation and collapse of lung regions. There is reduced surfactant in the alveoli due to destruction of pneumocytes by the virus, infiltration by white blood cells, such as neutrophils and macrophages, as part of the immune response, and oedema due to injury to blood vessels and leakage in response to proinflammatory factors released by the inflammatory cells. The fluid accumulation compresses the alveoli from outside and in combination with lack of surfactant, leads to their collapse. As a result, the surface area in the lung for gaseous exchange is reduced leading to hypoxia and dyspnea.
Phase 4: ARDS, The Cytokine Storm, and MOF: The critical illness in COVID-19, frequently develops in a period of about 10 days, though it can occur suddenly in a small proportion of those with mild or moderate disease. There occurs formation of fibrin clots in the alveoli and fibrin-platelet microthrombi in the small blood vessels in the lungs affecting gaseous exchange at the alveolar level. The cytokines, such as IL1, IL6, and TNFα damage and dilate the vessel walls, making them more permeable and may lead to cardiovascular shock. The angiotensin converting enzyme 1 (ACE1), in response to infection, leads to excess availability of angiotensin-2 from angiotensin-1, resulting in pulmonary vasoconstriction and leaky blood vessels.
The Recovery or Convalescence Phase
The usual recovery time for mild COVID-19 is about two weeks and three to six weeks for severe disease. However, the recovery is variable and depends on Constitutional factors such as patient’s age and pre-existing comorbidities in addition to the severity of the disease. The studies in the U.S. show that only 39% of those who had been hospitalized reported a return to baseline health by 14-21 days after diagnosis [8]. Similar findings have been reported from the European studies. In a study of 143 patients hospitalized for COVID-19, only 13% were symptom-free after a mean period of 60 days following disease onset [9]. The remaining 87% patients reported persistence of symptoms such as, fatigue, cough, dyspnoea, joint pains, and chest pain following discharge from the hospital, with over 55% patients continuing to experience three or more symptoms. As measured by the EuroQol visual analog scale, a decline in quality of life (QOL) as was noted in about 44% patients. The pneumonia-like manifestations may persist for several weeks in immunosuppressed patients. Even the patients with milder infection can suffer with prolonged symptoms. A recent survey showed that about 65% of those infected returned to baseline health by 14-21 days after diagnosis [10]. The persisting symptoms with delayed recovery include cough (43%), fatigue (35%) and rarely fever and chills in those with prior mild infection.
Pathophysiology of Lung Damage in Covid-19
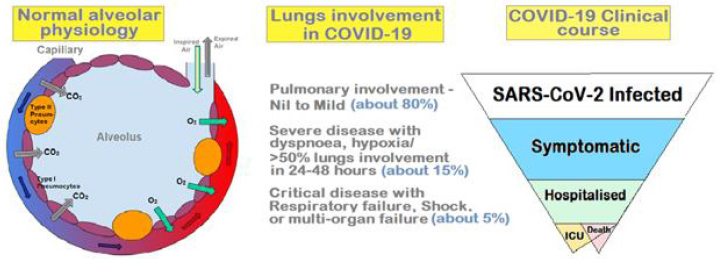
SARS-CoV-2 Infection: Challenge to Normal Physiology: The alveoli are the basic functional units in lungs, where the oxygen from inspired air is exchanged with the carbon dioxide, which is expired subsequently. Normally, there is a tight connection between the alveolar type I (AT-I) cells and the capillaries. The AT I cells are thin and flat cells which line the alveolus, interspersed by alveolar type II (AT II). The type II pneumocytes secrete surfactant, which lines the walls of alveoli and prevents them from collapsing and sticking to each other at the end of a respiratory cycle, when the air pressure inside the lungs drops in the expiration phase. COVID-19 infects alveolar cells (pneumocytes) leading to damage of the alveolar wall and the lining of the alveolus and capillaries. In addition, there occur microthrombi, which block the micro-vessels. The debris in form of plasma protein accumulates on the alveolus wall and thickens the lining leading to the impaired gaseous exchange and oxygen transfer to the red blood cells. Simultaneously, there occurs loss of surfactant as the infection affects the surfactant producing cells, which are rich in ACE2 receptors [11]. Other contributory factors for loss of surfactant are environmental factors, like air pollutants and smoke, and a hyperactive immune response. The direct damage of the virus-infected cells as well as cytokine hyper-response reduces the availability of surfactant in the alveoli leading to their collapse at the expiratory phase, manifesting as considerable stress on respiratory muscles at subsequent inspiratory phase evidenced as dyspnoea and hypercapnia [12].
Inter-Relationship of Pathophysiology and Clinical Spectrum
The respiratory involvement in COVID-19 may be mild to moderate to severe (Figure 2). Depending on the extent of underlying pathophysiology in the mild disease there are infected AT I and II cells and presence of inflammatory cells and secretion of cytokines leading to reduced surfactant, vasodilatation, and reduced gaseous exchange leading to hypercapnia and hypoxia. Whereas, in a more severe disease, there occur increased interstitial fluid, widespread alveolar collapse, accumulation of protein and cellular debris and fibrosis leading to severely compromised gaseous exchange and inability to maintain tissue oxygenation in various organs. To prevent redundant blood circulation through the collapsed alveoli, there occurs constriction of blood vessels supplying these alveoli. Depending on the severity of damage, the respiratory muscles work to handle the respiratory stress, but eventually tire, leaving the gaseous exchange compromised. In COVID-19, not only the respiratory cells richly endowed by ACE2 receptors are affected, the lung vasculature having ACE2 receptors is also infected and damaged impairing the compensatory blood redistribution from collapsed alveoli, resulting in significant redundant deoxygenated blood flow [13]. Further, the associated hypercoagulability with COVID-19, further hampers gaseous exchange in lungs and oxygen supply to various tissues and vital organs [14].
In addition, following infection, AT II cells release inflammatory signals to recruit immune cells including macrophages which release cytokines causing vasodilatation leading to increased permeability and fluid accumulation. The increased fluid dilutes surfactant and triggers alveolar collapse, leading to decreased gaseous exchange and increased work of breathing. The neutrophils recruited to the inflamed areas also release reactive oxygen species (ROS), which cause destruction of the infected AT I and AT II cells leading to widespread alveolar collapse precipitating ARDS. Finally, the protein rich fluid and debris enter the blood stream and precipitate systemic inflammatory response syndrome (SIRS), associated with septicaemia, shock, DIC, and MOF [15].
Host Immune System and SARS-CoV2 Infection
The viral genome typically consists of 6 open reading frames (ORFs), of which the ORF1a and ORF1b produce polypeptides, pp1a and pp1b, respectively, which form non-structural proteins (NSPs). The remaining ORFs synthesise of various structural proteins (SPs) including the spike and envelop proteins. The SPs induce immune response whereas NSPs appear to modify the host immune response. The SARS-CoV2 infection induces both innate and adaptive immune response and leads to infiltration by macrophages and T cells which release various pro-inflammatory cytokines/chemokines and lead to molecular changes in the lung tissue microenvironment. The pro-inflammatory cytokines induce DNA damage, excessive production of angiogenic molecules (VEGF, IL-8, NO), ICAM-1 and VCAM-1, production of ROS and reactive nitrogen species (RNS), and alterations in cellular proteins and stimulation of cell proliferation and inhibition of apoptosis. The cytokines affect cells via activation of transcription factors like STATs, NF-kB, and AP-1 by binding to their specific receptors and subsequent activation of intracellular kinases like Janus activated kinase (JAK), phosphatidylinositol-3-kinase (PI3K)/Akt, and MAP kinases. There can occur a sudden cytokine-burst, the cytokine release syndrome (CRS), leading to exacerbation of pneumonia and respiratory failure, and multiple organ dysfunctions. This is also associated with rapid progression of the viral infection, destruction of the immune system, and increased susceptibility to secondary infections.
There is another aspect of increased plasma level of TNFα, IL-6 and IL-1β, which are present in COVID-19 patients. TNFα is a major associated pro-inflammatory cytokine, having a potential for DNA damage and cellular transformation through ROS in inflammation-associated carcinogenesis. The IL-6 promotes several pro-oncogenic pathways and genetic expression for the cell cycle progression and regressing of apoptosis, and suppression the host antitumor immune responses. Whereas other cytokines such as IL- 1α, IL-1β, and IL-17, elevated in COVID-19 disease may involve the regulation of inflammation-related carcinogenesis. Further, IL-1α has been related to increased cell proliferation and angiogenesis, and IL-1β plays an important role in proliferation of epithelial cells in lungs. The COVID-19 patients, thus, have an increased propensity to develop cellular metaplasia in due course. In this respect, the similarity and overlap in CT features between the COVID-19 illness and lung cancer progression have been highlighted. The widespread GGO findings in CT images of COVID-19 patients have raised the probability of developing lung cancer. Thus, there is a need for follow up at regular interval for early detection of any kind of pre-neoplastic lesions [16].
The Long-Term Lung Damage in COVID-19
As COVID-19 is a relatively new disease, the long-term effects of COVID-19 in those who recover, are still not known, but various recent observational case and cohort studies continue to provide data. Based on initial case studies from those with moderate-severe disease and those suffered with pneumonia, the initial damage to the lungs can persist leading to decreased lung function and impact the activities of daily living (ADL). Pulmonary fibrosis is one of the major complications of severe COVID-19 and even those fully recovered symptomatic patients, may have some long-term lingering effects persisting for several months. Depending on the severity of respiratory inflammation and damage, presence of comorbidities, duration of injury and ill-defined genetic factors, the progressive fibrosis may lead to compression of lung tissue and damage of pulmonary microvasculature. The chronic inflammation leads to epithelial damage and fibroblast activation, being the major causative factor for pulmonary fibrosis. In addition, the lope-sided regeneration and the presence of fibroblasts and the excessive deposition of hyaline, collagen, and other extracellular matrix proteins lead to alveolar damage and scarring in lungs. In case of about 23% of the recovered SARS patients, there was seen reduced lung exercise capacity and pulmonary function a year after infection [17]. The COVID-19 patients with moderate/severe symptoms are likely to have a similar level of long-term reduction in lung function. Thus, depending on the severity of the disease, extensive and longlasting damage to the lungs can occur, which may persist well after the infection.
Autopsy Findings in Lungs of COVID-19 Patients
A Post-mortem study involving 41 people died from COVID-19 in Italy has revealed extensive damage, distortion of the pulmonary structure with scarring and massive blood clots in the arteries and veins [18]. Out of 41 cases, all 41 patients had extensive lung damage, while 36 of 41 (88%) had massive abnormal blood clotting in the lung arteries and veins. Further, in the study, nearly 90% of the lungs showed the fusion of smaller cells into giant single cells with many nuclei. The fused cells, or syncytia, are caused by SARSCoV- 2 S protein, which stimulates the fusion of infected cells with normal lung cells, leading to structural changes, inflammation, and abnormal blood clotting. The extent of damage to lungs in severe COVID-19 is, thus, enormous especially in those that are clinically vulnerable [19]. Autopsy findings reveal extensive lung damage, with firm, heavy and rubbery lungs with bilateral haemorrhagic edema, pleural effusion as well as signs of extensive shock characterized by variegated appearance of the liver and kidneys [20]. There is extensive endothelial injury associated with immune cell infiltration including T-lymphocytes and megakaryocytes. In the pulmonary vessels there is widespread thrombosis with microangiopathy and alveolar capillary microthrombi in addition to neovascularization and angiogenesis. The SARS-CoV-2 genome has been found in respiratory cells, cells lining the blood vessels, and the syncytia, in these patients. These histopathologic changes are pathognomonic of COVID-19 pneumonia and the persistence of the abnormal cells and the virus-infected cells may be linked with continuance of ongoing viral replication and organ damage, and persistence of long Covid symptoms in those recovered from the disease.
The Clinical Correlates of Lung Involvement
Clinical Presentation of COVID-19
Depending on the severity of damage, the respiratory involvement may appear as asymptomatic, mild to moderate respiratory illness, severe bronchopneumonia, or acute respiratory distress syndrome (ARDS).
Asymptomatic COVID-19 Patients
A large proportion of healthy individuals, over 40%, found positive for SARS-CoV-2, do not exhibit obviously significant symptoms. They are, however, able to transmit the disease. The majority of these asymptomatic patients are from younger age group. But the asymptomatic patients not showing any signs of lung damage, may suffer subtle changes, potentially predisposing them for complications in future. In these asymptomatic COVID-19 patients, there have been shown lung abnormalities on chest CT scans. This is exemplified by the outbreak on Diamond Princess cruise ship, at beginning of the COVID-19 pandemic in February 2020, where 73% of those positive were asymptomatic, of which 54% showed lung opacities (ground-glass opacities; GGO) indicating alveolar edema, inflammation, and fibrosis in the lungs. This may be an age-dependent effect and may occur to lesser extent in younger patients.
Symptomatic COVID-19 Patients
In most people, COVID-19 leads to very mild, mild, or moderate clinical manifestations. The variance in clinical presentation may be attributed to amount of viral load, age, pre-existing health conditions, genetic constitution, ethnicity/demographics, lifestyle, and environmental factors. The majority of pathological and imaging data come from hospitalized COVID-19 patients rather than non-severe symptomatic patients who may not visit a medical facility for diagnostic workup or treatment [21].
Severe COVID-19 Illness and Pneumonias
The patients with severe disease manifesting as pneumonia and ARDS, exhibit extensive alveolar damage, capillary congestion, necrosis of AT I and II pneumocytes, and interstitial and alveolar edema. Simultaneously, there is AT II pneumocyte hyperplasia, squamous metaplasia with atypia and platelet-fibrin thrombi in small arterial vessels, and increased D-dimer levels. As these changes hinder the pulmonary function, and the higher the impairment, the higher is disease severity and mortality. In severe disease, micro-thrombosis and associated ischemic events are common and the ARDS occurs due to the compromised gaseous exchange and the vascular insult to the alveolar architecture. The COVID-19 pneumonia presents with severe hypoxemia and altered respiratory mechanics [22]. The alveolar infiltration and the vascular insult are common factors responsible for the respiratory distress [23]. Accompanied with by hyper-activated coagulation cascade, with widespread micro- and macro-thromboses in the lung and in other organs and elevated serum D-dimer levels, this is associated with adverse outcomes. Simultaneously, the endothelial damage disrupts pulmonary vaso-regulation, promotes ventilationperfusion mismatch, and promotes thrombogenesis. In the lungs, the ARDS directly impacts the alveoli and other tissues leading to extensive damage with scarring and fibrosis.
COVID-19 Related ARDS and Respiratory Failure
With the progression of COVID-19 pneumonia, increasing number of alveoli become affected and filled with exudative fluid clinically manifesting as shortness of breath. Further progression leads to ARDS, a form of lung failure requiring respiratory support to improve gaseous exchange. The ARDS is a serious and potentially fatal complication, and in the survivors may have lasting symptoms due to pulmonary fibrosis and scarring. It is helpful to assess and categorize the COVID-19 patients as per symptoms and signs of the respiratory failure, as different ventilatory approaches are needed, depending on the underlying pathology [24]. In general, the severe COVID-19 illness presents with two types of respiratory syndromes, the type 1 without ARDS, and type 2 with ARDS [25]. The two types may have overlapping characteristics. Further, during the early phases of respiratory decompensation, the high transpulmonary pressures associated with spontaneous vigorous inspiratory effort may contribute to lung injury, called the patient self-induced lung injury [P-SILI) resulting in deterioration of respiratory failure.
Type 1 or L Type: These patients present with near-normal pulmonary compliance with isolated viral pneumonia. The hypoxemia is associated with respiratory system compliance > 50 ml/cmH2O and mainly due to the hypoxic pulmonary vasoconstriction and impaired regulation of pulmonary blood flow. There is, thus, severe hypoxemia due to ventilation/perfusion (VA/Q) mismatch and high PEEP and prone positioning may not improve oxygenation through recruitment of collapsed areas but redistribute pulmonary perfusion and improve the VA/Q relationship. In fact, long-term prone positioning/supine cycles is of little benefit in these patients. The PEEP levels should be kept lower in these patients with high pulmonary compliance. Respiratory rate should not exceed 20 breaths/min and doing too much should be avoided.
Type 2 or Type H: These patients present with decreased pulmonary compliance and severe hypoxemia is associated with compliance values < 40 ml/cmH2O, indicating significant ARDS. The type 2 respiratory syndrome is seen in 20–30% of COVID-19 patients admitted to the ICU and the patients may transit to a clinical picture characteristic of ARDS, with extensive CT consolidations, high elastance (low compliance), and higher lung weight as assessed by CT scans. These patients belong to type H and respond to high PEEP by increasing aerated lung size by recruiting previously collapsed lung units, but the high transpulmonary pressure induces stress across the lung that may not be well-tolerated by these patients. A relatively low tidal volumes, together with modest hypercapnia, facilitate the goal of minimizing ventilator-induced lung injury (VILI) and prone positioning are helpful. Following onset of respiratory distress, patients initially retain relatively good compliance despite compromised oxygenation, may not appear overtly dyspnoeic, and are termed type L. The infiltrates at this stage are limited in extent and characterized by a ground-glass pattern signifying interstitial edema, and lower lung weight as assessed by CT scans. There are chances that the clinical condition may stabilize at this stage without further deterioration. But, if pneumonia and lung edema increase in the type L patient, either because of the disease inciting inflammation and/ or P-SILI, the type H phenotype may progressively develop. Over time, the unchecked viral disease promotes inflammation and local and generalized thrombogenesis, intense cytokine release, right ventricular overload, and systemic organ dysfunction. In the advanced state, it is advisable to apply a conventional lung-protective strategy: higher PEEP (≤15 cm H2O), lower tidal volume (6 mL/kg), and prone positioning while minimizing oxygen consumption. A daily check of coagulation parameters, in particular D-dimer levels, should be performed in both the type 1 and the type 2 patients and judiciously anticoagulated when indicated [22].
Ct Imaging in Covid-19 And Post-Covid Follow Up
CT Imaging During Acute COVID-19 Illness
The computed tomography (CT) is an important tool for early diagnosis and monitoring of the affliction of lungs in COVID-19. The ground glass opacities (GGOs), dense opacities obscuring underlying vessels and bronchial walls, are common in COVID-19. Studies have shown a higher frequency of multifocal, bilateral, peripheral, and nonspecific distribution of GGO with sub-segmental patchy consolidations in SARS-CoV2-infected individuals compared to controls [26]. Another study highlighted that more than 50% of the SARS-CoV2-infected patients have significant ground-glass and consolidative opacities on chest CT scans [27]. The chest CT scans can be helpful for the diagnosis as well as for monitoring the prognosis. There are peripheral lung ground-glass opacities seen in CT imaging of the chest. Peripheral pulmonary vascular changes are less well characterized. The effects of SARS-CoV-2 infection on the alveolar architecture manifesting as GGOs depend on the severity of the infection and its effects on the immune system, and imply partial collapse of alveoli, partial filling of air space, and interstitial thickening. The severity of the disease is related to the extent of lesions on initial chest CT scans and the abnormalities on CT scans have been related to the disease course [28]. In the course of the disease, the GGO occurs from the beginning of COVID-19 and tends to decrease after 14 days, whereas consolidations appear around day 9, followed by fibrosis after 14 days.
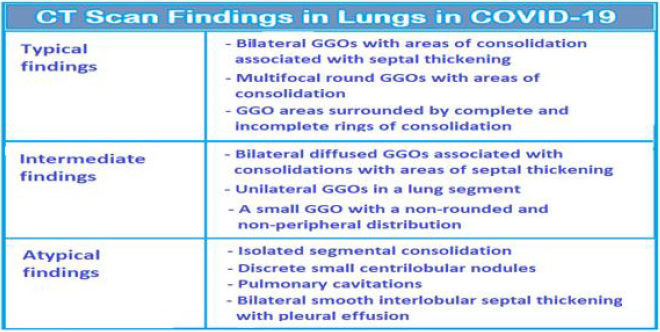
There are typical, indeterminate, and atypical findings on CT Imaging in COVID-19 (Figure 3). The typical findings are bilateral GGOs with areas of consolidation, associated with septal thickening. The scans may show multifocal round GGOs with areas of consolidation or the GGO areas surrounded by complete and incomplete rings of consolidation. The indeterminate findings are bilateral diffused GGOs associated with consolidations with some areas of septal thickening or unilateral GGOs in a lung segment or a small GGO with a non-rounded and non-peripheral distribution. Whereas the atypical findings are isolated segmental consolidation, discrete small centrilobular nodules, lung cavitations and bilateral smooth interlobular septal thickening with pleural effusion [29]. In general, at time of initial presentation 88% patients show GGOs with bilateral involvement, 80 % show posterior distribution, 79 % multi-lobar involvement, and 76% have peripheral distribution, whereas only 32% have consolidation on initial presentation [30]. The pulmonary fibrosis is a major consequence of SARS-CoV-2 infection and primarily results due to direct alveolar damage and as the fallout of ARDS. The effect of pulmonary fibrosis and ongoing damage in the lung is also observed later in the absence of the infection and has been attributed to the persistent hyperinflammatory state, with the elderly being at higher risk. Further, SARS-CoV2 is a cytopathic virus that can induce host cell lysis following infection. The virus led pyroptosis causes apoptosis along with excessive immunologic response, resulting in increased IL-1β level and cytokine storm.
The CT findings of GGO have also been correlated with increased expression of ACE2. There take place different stages of pulmonary tissue damage in the lungs of COVID-19 patients with the appearance of GGO, and include consolidation, reticular pattern, crazy paving pattern, air bronchogram including the airway changes, pleural changes, sub-pleural curvilinear line, and finally, fibrosis and scarring seen on CT images.
CT Imaging for The Long-Term Follow Up
A long-term study highlights that in the SARS infection, the fibrosis-like findings on CT in recovering patients diminish over time [31]. It is expected that similar prognosis may be possible in convalescent COVID-19 patients [32]. In the Post-COVID-19 patients, the follow up CT scans may reveal probable lifetime lung damage in about one-third of the patients. About 20-30% of patients who have suffered mild disease may have persisting diminished lung function and exercise capacity, whereas in 55% of patients with critical disease may continue to have the lung function impairment three months following hospital discharge. Further, there has been shown a definite correlation between initial chest CT scan involvement and impaired PFT [33]. The lung function, exercise capacity, persistent radiologic abnormalities, and quality of life data have been related to radiologic lung involvement at admission. In fact, the later radiological and functional sequelae have been related to initial lung involvement on CT scans. On chest CT, the main abnormalities are ground glass opacities at hospital admission and fibrosis 3 months later, and the patients remained largely disabled 3 months after discharge in terms of lung function, exercise capacity, and QoL. Radiologic impairment may improve following recovery, but normalization is seen only in a small number of patients. In general, about 12%-23% COVID-19 hospitalized patients show restrictive pattern on PFT one month after discharge [33]. In the study, the exercise capacity was still poor 3 months after ICU discharge and decreased QoL was associated with severity parameters during ICU admission and chest CT abnormalities on admission.
Pan et al. analysed 21 patients (six men and 15 women aged 25–63 years) with confirmed COVID-19 with a total of 82 chest CT scans and reported that in patients recovering from coronavirus disease 2019 (without severe respiratory distress during the disease course), lung abnormalities on chest CT scans showed greatest severity approximately 10 days after initial onset of symptoms [34]. The follow up CT studies have revealed that the lung damage may persist in post-COVID-19 patients and over onethird of recovered patients follow-up CT scans after six-month show pulmonary fibrosis [35]. In a follow-up CT scans study involving 114 patients who recovered from severe COVID-19 pneumonia, about 62 percent still showed evidence of abnormalities on the scans after 6 months, whereas about 35 percent had fibrosis-like features indicating potentially permanent damage, such as honeycombing patterns and parenchymal bands. In all, about up to 63 percent of those suffered with ARDS had lingering lung alterations [35].
Covid Follow Up: Links with Neoplastic Lesions
The SARS-CoV-2 Infection causes molecular changes and recruitment of inflammatory cytokines/chemokines in lung tissue microenvironment [16]. As well known, several pro-inflammatory cytokines are involved inflammation-associated neoplastic alterations through various molecular and metabolic pathways, and ROS. The widespread GGO findings in CT images of COVID-19 patients have raised the probability of developing lung cancer and require a regular follow up for early detection of any pre-neoplastic lesions.
The Disease Continuum from Covid-19 To Long Covid
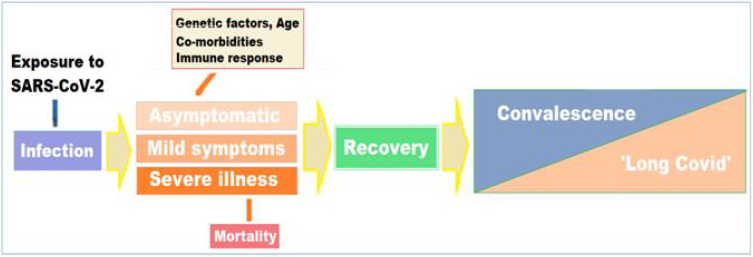
Factors Influencing the Pulmonary Manifestations During Disease Course: It is becoming clear that apart from the advanced age and pre-existing conditions, such as diabetes, cardiovascular, pulmonary, and renal diseases, certain constituent factors render some patients more vulnerable to the more severe forms of the disease including pneumonia and ARDS. In addition, certain other known factors including the constituent factors and several still unknown factors may influence the COVID-19 manifestations, its course, and later the convalescent phase. From the clinical perspective, knowledge of host constituent factors, including the genetic variations, could lead to improved care for patients with COVID-19 during the acute phase of the disease as well as during the convalescence and ‘long Covid’ phase. The host genetic factors have been linked to the variable clinical manifestations of the disease following exposure to the virus at the individual level as well as in various population groups [36]. In fact, a model to understand several related factors such as age, associated comorbidities and genetic factors including human genomic variants linked to COVID-19 outcomes can be conceived as a continuum of the disease course [Figure 4].
The genetic factors and pathways related to the disease manifestations involve specific deviations in the genes and pathways responsible for inter-individual COVID-19 susceptibility and response. To track the acute as well as long-term impact of the disease on health, the project called the COVID Human Genetic Effort, aims to find genetic variants that compromise the immune systems and render certain individuals more vulnerable to the risk of developing Covid-19 [37]. In addition, the project aims to document the genetic variations which make some individuals resistant to the SARS-CoV2 infection. In nutshell, the project aims to discover, monogenic inborn errors of immunity (IEI) underlying severe forms of COVID-19 in previously healthy individuals, monogenic variations which make certain individuals resistant to the SARS-CoV2 infection despite repeated exposure, and decipher the molecular, cellular, and immunological mechanisms by which they cause resistance to viral infection or predisposition to a severe form of the disease.
The Covid-19 Delayed and Long-Term Effects
There is a growing evidence of persisting multisystem effects of COVID-19, indicating substantial continuing morbidity after resolution of the infection [9]. While most people recover quickly and completely from the virus, persistent manifestations, especially respiratory symptoms are frequently seen and reports from cohort studies suggest that one in three people may not fully recover several weeks after initial illness and a smaller but still substantial proportion continue to have symptoms and disabilities that persist for months [38]. Further, the long-term effects of COVID-19 are seen in the younger population also, though the risk of ‘long Covid’ increases with age. People who suffer with severe form of the disease, experience long-term inflammation and damage in lungs, heart, immune system, brain, the vasculature, and other organs. These long-term effects may last for months and years. The ‘long Covid’ is not contagious and results due to the body’s response to the virus infection continuing beyond the initial illness. Most Covid-19 patients recover within few weeks without significant complications. In others, often the persisting symptoms may go unnoticed, as they are vague and nonspecific. But some patients, even those who had mild versions of the disease, including the younger people afflicted with mild or asymptomatic disease and those who did not suffer with serious disease or require hospitalization, may continue to experience symptoms after their initial recovery [39]. These patients have been described as long haulers and the clinical condition due to persisting or continuing symptoms has been called post-COVID-19 syndrome or ‘long COVID-19’. The clinical condition encompasses a delayed convalescence or recovery, persistence of symptoms, and emergence of symptoms related to the organs involved and damaged, and incapacitating complications and sequelae (Figure 5).
The long-haul COVID patients carry their symptoms well beyond the normal course of recovery lasting for weeks and months or longer. Further, of various facets of the disease, the long- Covid syndrome may in due course prove to be the most difficult to deal with. These symptoms are often varied and relatively common and may defy a COVID-related diagnosis. Several patients who are expected to recover, continue to suffer for a variable period of weeks and months with various general symptoms such as breathlessness on exertion, fatigue, dizziness, memory lapses and other cognitive issues, digestive disorders, erratic heart rates, headaches, fluctuating blood pressure, and muscular and joint pains, which are often considered by the patient himself and family members as related to the weakness developed following the disease. These patients report weeks and months-long symptoms affecting lungs, heart and other organs and given the multitude of COVID-19 cases worldwide, the prevalence of ‘long Covid’ is expected to be substantial and likely to increase with the recurrent outbreaks of the disease. Furthermore, the ‘long Covid’ with debilitating and prolonged illness may have profound impact on health of people, their social life and livelihoods, and the economy.
The Presentation of ‘Long Covid’ Syndrome
The occurrence of persistence or appearance of symptoms related to ‘Long Covid’ are being increasingly reported. Over the past few months evidence has mounted about the significant longterm effects of COVID-19 and it is estimated that there may be more than five million people with ‘long Covid’ symptoms. The worldwide distribution of COVID-19 suggests that many of those people are currently living and experiencing the misery in the U.S. The ‘long Covid’ is neither well-defined nor well understood, partly because the related research is still in its infancy. The symptoms persist or develop outside the initial viral infection, and the duration and exact pathogenesis are unknown. Further, they vary from vague to severe incapacitating symptoms, and there is often a relapsing and remitting pattern. There is little known about the prevalence, risk factors, or probability to chart the protracted course early in the course of the disease. It appears that the etiology of the syndrome is multifactorial and may involve unbound immune responses, cardiopulmonary or systemic inflammation, vascular inflammation and coagulation disorders, and a direct cellular damage from viral replication during acute illness. The syndrome has vastly emerged from self-reporting but is a real clinical entity with the chronic health manifestations, and characterised by symptoms of fatigue, headache, dyspnoea, and anosmia and likely to increase with age, higher BMI, and female sex. Further, as deciphered from various studies, experiencing more than five symptoms during the first week of illness is associated with ‘long-Covid’ [40].
In a study with online survey data involving over 4,000 Covid-19 patients, about 13.3% of all ages suffered with the symptoms lasting >28 days, whereas 10% of those aged 18-49 years had the related symptoms 4 weeks after acquiring the infection. Further, 4.5% patients of all age-groups suffered with the symptoms for more than 8 weeks, and 2.3% of all ages for more than 12 weeks. The study was conducted by health-science firm Zoe Global Limited in conjunction with Biomedical Research Centre based at GSTT NHS Foundation Trust and supported by the UK Research and Innovation [41]. The analysis and inference derived from similar studies could be used to identifying individuals with ‘Long-Covid’ may help to reduce long-term complications and sequelae and planning health education, guidance, and rehabilitation services [42].
Diagnosis and Manifestations of ‘Long Covid’
As a matter of fact, medical advice should be routinely sought for all the patients having delayed recovery and persistence or emergence of symptoms. There is a multitude of adverse physical and mental health effects due to ‘long Covid’ and these afflictions may last for an indefinite period. According to a study published in the Lancet, which included 1,733 people tested positive for Covid-19 and followed for 4 months, documented that more than 75% of the people who were hospitalized for COVID-19, continued to suffer with at least one symptom for 6 months after recovery. Further, it was noted that about 76% of them experienced lingering symptoms of COVID-19 long after being cured of the illness (17). In another recent study, Carvalho-Schneider et al. followed-up of 150 adults with only mild to moderate COVID cases for two-month and found that two thirds of them were still experiencing symptoms, most commonly shortness of breath, loss of smell and taste, and/ or asthenia and fatigue [43]. Another study by Italian researchers, covering 143 COVID patients who had been discharged from the hospital, found that only about one in eight was completely free of symptoms 60 days from the beginnings of the illness [44]. The King’s College London study, one of the largest surveys so far, reported that around 10 percent of patients had persistent symptoms for one month, with 1.5 to 2 percent having sustained symptoms at three months. Further, the study documented that long COVID was twice as common in women as men, and the older people, and those with more than five symptoms during their first week of illness were more likely to develop ‘long Covid’ [41].
Management Of ‘Long Covid’ Syndrome
The Clinical Workup and General Guidelines: There is a Covid healthcare continuum for management of pulmonary manifestations of COVID-19 and long Covid (Figure 6). It encompasses diagnostic workup, clinical follow up, investigational workup, followed by assessment of organ damage and treatment which involves pharmacological as well as non-pharmacological treatment in form of holistic care (Figure 6).
General Clinical Guidelines
a) Chest Pain: Non-specific chest pain is common in postacute covid-19. It could be musculoskeletal, unexplained nonspecific chest pain, or due to a cardiovascular condition. Any persistent or recurrent pain in chest in setting of post-COVID convalescence requires meticulous workup.
b) Respiratory Symptoms: A degree of breathlessness is common during acute covid-19 and convalescence. There could be worsening breathlessness and severe breathlessness may require hospitalization. In general, the breathlessness tends to improve with breathing exercises and guided pulmonary rehabilitation.
c) Thromboembolism: Covid-19 is an inflammatory and hypercoagulable state, with an increased risk of thromboembolic events including pulmonary thrombo-embolism. The hospitalised patients, in general, receive prophylactic anticoagulation followed by anticoagulant therapy after discharge in the high-risk patients for an extended period.
d) Other Symptoms: Other symptoms such as anxiety, stress, and insomnia are common and may have a bearing on cardopulmonary status and care. The elderly patients, in addition, are more prone to risk of sarcopenia and malnutrition.
Investigations during ‘long Covid’ Follow up
a) Blood tests - Anaemia should be excluded. Lymphopenia is a feature of severe, acute Covid-19 illness, whereas leucocytosis may denote infection or inflammatory response.
b) The biomarkers - They include C reactive protein, d-Dimer, LDH, and ferritin indicative of inflammation and continuing prothrombotic state.
c) An ECG and Chest X Ray - at 12 weeks or earlier for new, persistent, or progressive symptoms. Cardiac echo, CT scan chest or MR scan may be required.
d) Further studies and research are likely to refine the indications and interpretation of diagnostic and monitoring tests in follow-up of ‘long Covid’.
Supportive Treatment and Rehabilitation: The ‘long Covid’ syndrome has multi-system involvement and unpredictable course with variable presentation depending on unmodifiable factors such as race, age and sex, and modifiable factors like comorbidities and lifestyle, and certain unknown factors. Following clinical and investigational assessment, the patients should be managed according to their clinical manifestations, extent of organ damage and associated complications. In case of suspected pulmonary and cardiac involvement, the restriction of physical activity advised, followed by periodic assessment. An early resumption of physical activity and exercise in the presence of pulmonary dysfunction may also be associated with both increased morbidity and mortality [45]. Although there are no firm data on COVID-19 recovery, many reports indicate that it may takes weeks until people with even moderate infections are back to baseline. If the pulmonary function evaluation is unremarkable and there are no further cardiopulmonary symptoms, the patient can slowly and gradually resume physical activity, beginning with short walks. Current recommendations for resuming physical activity following COVID infection recommend no exercise for 2 weeks from positive test, close monitoring for symptoms, slow resumption of exercise for asymptomatic post-COVID patients, those with Mild Post-COVID symptoms are advised rest for 2 weeks, clinical evaluation, with consideration for X ray chest, ECG, and echocardiogram before resuming exercise. Whereas those having suffered with severe COVID-19 illness need to undergo complete cardiopulmonary evaluation during hospitalization and 2 weeks after discharge, close monitoring and supervised slow resumption of activity [46].
Activity Guidance and Occupational Rehabilitation
In general, all COVID-19 patients should be risk stratified following recovery before recommending a return to physical activity, which should be gradual, individualised, and based on subjective tolerance of the activity (Figure 7). It is noteworthy that apart from the severe cases and elderly, even those with mild disease and a proportion of people from all age groups may experience a prolonged recovery [47]. In general, a return to physical activity should be after at least seven days period free of symptoms, followed by two weeks of minimal exertion. As a rule, those with ongoing symptoms or history of severe covid-19 need cardio-pulmonary assessment before advising return to physical activity [48]. In practice, thus, for those with mild symptoms during the Covid illness and asymptomatic during convalescence period, there should be a phased return to physical activity with at least a week in between every phase. The phases have been outlined as -
Phase 1: Breathing exercises, mild stretching, and gentle walking.
Phase 2: Low intensity walking, mild household and gardening tasks, light yoga.
Phase 3: Moderate intensity aerobic and strength challenge.
Phase 4: Moderate intensity aerobic and strength challenge with coordination and functioning skills.
Phase 5: Return to regular exercise and physical activity pattern.
Usually, a light intensity activity is advised for initial two weeks. The Borg Rating of Perceived Exertion (RPE) scale is a subjective assessment of activity and physical work and helpful in guiding the progress through the phases of increasing physical activity. The patients must rate their subjective feeling of exertion, including shortness of breath and fatigue, on a scale from 6 (no exertion at all) to 20 - maximal exertion [49].
Conclusion: Evolving ‘Long Covid’ Scenario
Post-Covid Pulmonary Damage and Fallouts
The post-Covid pulmonary damage and fibrosis is characterized by unsuccessful reconstruction of the damaged alveolar epithelium, persistence of fibroblasts and excessive deposition of collagen and other extracellular matrix components, and the destruction of normal pulmonary architecture. Its progression in due course results in compression and destruction of normal pulmonary parenchyma, and damage to microvasculature. There takes place fibrin deposition in the alveoli and lung parenchyma, together with platelet thrombotic micro-clots in the pulmonary vessels. The elderly patients are more prone to viral-induced fibrosis due to immunosenescence. The recent reports show that serum levels of the various cytokines and growth factors including monocyte-1 chemoattractant protein (MCP-1), transforming growth factor β1 (TGF-β1), tumor necrosis factor a (TNF-α), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), interleukin- 1b (IL-1b) and interleukin-6 (IL-6), are overexpressed are also highly increased in COVID-19 patients. In addition, there occurs dysregulated release of matrix metalloproteinases, leading to epithelial and endothelial injury and uncontrolled fibroproliferation. From the clinical perspective, the COVID-19 incubation period and clinical phase involves 3 weeks. In this context, the post-acute Covid can be described as the illness extending beyond three weeks from the onset of first symptoms, and the chronic covid-19 as extending beyond 12 weeks. As being increasingly documented, about 10% of patients who have tested positive for SARS-CoV-2 virus remain unwell beyond three weeks, and a smaller proportion for further period.
These patients are known as the ‘long-haulers’ and the persisting affliction as ‘long Covid’ or ‘Post-Acute Sequelae of SARS-CoV-2 infection (PASC)’. In general, thus, the diagnosis of the ‘Long Covid’ or ‘Long haulers” should be entertained for various symptoms and signs that linger well beyond the period of convalescence in COVID-19 [50]. The most common of persisting signs and symptoms of post-Covid-19 illness related to respiratory system include extreme tiredness (fatigue) and giddiness, chest pain and tightness, palpitations, shortness of breath and Cough. The underlying disease state may vary from mild restrictive lung function to decompensated persistent chronic lung disease (Figure 8).
Pulmonary Sequelae and Therapeutic Options
Presently, there is no proven and fully documented effective therapeutic modalities for the treatment of post-inflammatory pulmonary fibrosis following COVID-19. But, as the disorder bears similarities in its pathogenesis with idiopathic pulmonary fibrosis including cytokine profiles, it has been suggested that the drugs useful in the treatment of IPF could be also beneficial for symptomatic and supportive therapy for COVID-19 patients. The most important factor in limiting pulmonary fibrosis is timely antiviral treatment and elimination of the causative agent. Though, currently, there is no fully substantiated therapeutic modality for the treatment of post-inflammatory pulmonary fibrosis after COVID-19, some therapies may be considered [51]. The anti-viral agents can reduce the viral load and the duration of viral pneumonia, and hence prevent and decrease the pulmonary fibrosis. Thus, the currently known antiviral agents like remdesivir and favipiravir may inhibit RNA replication due to high affinity to viral enzymes, reverse transcription or protein biosynthesis through premature termination or inhibition of nitrogenous bases synthesis. Remdesivir was found efficacious in inhibiting viral replication and restricting lung injury in the animal model of MERS infection. Favipiravir may be another possible treatment option. It has been shown that FPV is able to inhibit virus reproduction in vitro. Preliminary studies indicate viraemic clearance and improved radiological appearance following favipiravir treatment.
The rationale for the use of steroids in COVID-19 viral pneumonia is to decrease the host inflammatory response in the lungs, which can lead to the development of acute lung injury and ARDS. There are several reports suggesting that the use of spironolactone may be of significantly useful in fibrosis prevention. Tocilizumab, the monoclonal antibody against IL-6, has been claimed to have a beneficial effect on coronavirus patients with severe lung damage and elevated interleukin six levels. As the final option, lung transplantation has been considered and tried [52,53].
The ‘Long Covid’ Challenges and Solutions
The SARS-CoV-2 virus uses ACE2 receptors to cause interstitial lung damage followed by parenchymal lesions. ARDS is the common acute pulmonary complication of the COVID-19, whereas pulmonary fibrosis is the sequelae of both acute as well as chronic (‘long’) COVID-19 leading to permanent disability. As mentioned earlier in this review article, there is no proven and fully documented effective therapeutic modalities for the treatment of post-inflammatory pulmonary fibrosis following COVID-19, Given the global scale of the pandemic, the healthcare needs for patients with sequelae of COVID-19, especially in those with lung affliction are bound to increase in the near future. Following clinical and investigational assessment, the therapeutic strategy is likely to depend on the clinical manifestations, extent of damage in lungs and other organs, and associated complications. The challenge can be tackled by harnessing of existing healthcare infrastructure, development of scalable healthcare models and integration across disciplines with a combination of pharmacological and nonpharmacological modalities for improved physical and mental health outcomes for COVID-19 survivors in the long-run [54].
Figure 2: Gel Electrophoresis (TRIS-agarose 2%): DNA obtained from the cotton swabs and the marine sediments obtained with the integral protocol, with their positive PCR products of 1469 bp.
Figure 3: Analysis of 16S genomic DNA amplification with a bioanalyzer spectrophotometer.
(a) Gel imaging of the genomic DNA extracted with the integrated protocol from 11 SPFS sample pools.
(b) Trace of one of the samples shown in figure 3a. The samples analyzed here were used for library construction and sequencing on the IonTorrent S5 platform.
Figure 4: Sequencing information of one of the chips used to sequence the samples standardized on this study, showing the total reads, chip loading, and fragment length in base-pairs, in an Ion Torrent S5 genetic sequencer (Thermofisher Scientific).
References
- (2020) Max Planck Institute for the Science of Human History. COVID-19 is here to stay for the foreseeable future: Future of field-based sciences in the time of coronavirus.
- Oran DP, Topol EJ (2021) The Proportion of SARS-CoV-2 Infections That Are Asymptomatic. Annals of Internal Medicine.
- Pollitt KJG, Peccia J, Ko AI, Naftali Kaminski, Charles S Dela Cruz, et al. (2020) COVID-19 vulnerability: the potential impact of genetic susceptibility and airborne transmission. Human Genomics 14(1): 17.
- Wang C, Wang Z, Wang G, Johnson Yiu-Nam Lau, Kang Zhang, et al. (2021) COVID-19 in early 2021: current status and looking forward. Sig Transduct Target Ther 6: 114.
- Wölfel R, Corman VM, Guggemos W, Michael Seilmaier, Sabine Zange, et al. (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581: 465-469.
- Gozalbo-Rovira R, Gimenez E, Latorre V, ClaraFrancés-Gómezc, Eliseo AlbertbJavier, et al. (2020) SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J Clin Virol 131: 104611.
- Chen N, Zhou M, Dong X, Jieming Qu,Fengyun Gong, et al. (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 10223: 507-513.
- Tenforde MW, Kim SS, Lindsell CJ, Erica Billig Rose, Nathan I. Shapiro, et al. (2020) Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network-United States, March-June 2020. Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention 69.
- Carfì A, Bernabei R, Landi F (2020) For the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 324(6): 603-605.
- https://www.uchealth.com/en/media-room/covid-19/short-and-long-term-lung-damage-from-covid-19.
- Kommos, Constantin Schwab, Luca Tavernar, Johannes Schreck, Willi L Wagner, et al. (2020) The Pathology of Severe COVID-19-Related Lung Damage. Dtsch Arztebl Int 117(29-30): 500-506.
- Aguiar, Johannes Alexander Lobrinus , Manuel Schibler, Tony Fracasso , Christelle Lardi (2020) Inside the lungs of COVID-19 disease. Int J Legal Med 134(4): 1271-1274.
- Guo J, Huang Z, Lin L, Jiagao Lv (2020) Coronavirus Disease 2019 (COVID‐19) and cardiovascular disease: A Viewpoint on the Potential Influence of Angiotensin‐Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. 9 Apr 2020, Journal of the American Heart Association 9: e016219.
- Jain U (2020) Effect of COVID-19 on the Organs. Cureus 12(8): e9540.
- Frizelli, Domenico Tuttolomondo, Marina Aiello, Maria Majori, Giuseppina Bertorelli, et al. (2020) What happens to people's lungs when they get coronavirus disease 2019? Acta Biomed 91(2): 146-149.
- Sadhukhan P, Ugurlu MT, Hoque MO (2020) Effect of COVID-19 on Lungs: Focusing on Prospective Malignant Phenotypes. Cancers (Basel) 12(12): 3822.
- Huang C, Huang L, Wang Y, Xia Li, Lili Ren, et al. (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397(10270): 220-232.
- Bussani R, Schneider E, Zentilin L, Chiara Collesi , Hashim Ali, et al. (2020) Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. Lancet-Ebio Medicine 61: 103104.
- Ackermann M, Verleden SE, Kuehnel M, Axel Haverich, Tobias Welte, et al. (2020) Pulmonary Vascular Endotheliitis, Thrombosis, and Angiogenesis in Covid-19.
- Carsana, Roberta Simona Rossi,Alessandro Pellegrinelli, et al. (2020) Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis 20(10): 1135-1140.
- (2021) Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19).
- Gattinoni L, Chiumello D, Rossi S (2020) Editorial - COVID-19 pneumonia: ARDS or not? Critical Care 24: 154.
- Marini JJ, Gattinoni L (2020) Management of COVID-19 Respiratory Distress. JAMA323(22): 2329-2330.
- Cascella M, Rajnik M, Aleem A, Scott C. Dulebohn; Raffaela Di Napoli (2021) Features, Evaluation, and Treatment of Coronavirus (COVID-19).
- Torres Acosta MA, Singer BD (2020) Pathogenesis of COVID-19-induced ARDS: implications for an aging population. European Respiratory Journal 56 (3): 2002049.
- Yang W, Sirajuddin A, Zhang X, Guanshu Liu, Zhongzhao Teng, et al. (2020) The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol 30(9): 4874-4882.
- Fu F, Lou J, Xi D, Yan Bai, Gongbao Ma, et al. (2020) Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur Radiol 30(10): 5489-5498.
- Kong W, Agarwal PP (2020) Chest Imaging Appearance of COVID-19 Infection. Radiology: Cardiothoracic Imaging 2(1).
- De Pádua Gomes FL, Kaiser FE, Nunes U, Bruna Melo Coelho Loureiro, Yuri Costa Sarno Neves, et al. (2020) Imaging findings in COVID-19 pneumonia. Clinics 75: e2027.
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients. AJR Am J Roentgenol 215(1): 87-93.
- Zhang P, Li J, Liu H (2020) Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res 8.
- Wells AU, Devaraj A, Desai SR (2021) Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties. Radiology 299: E216-E218.
- Truffaut L, Demey L, Bruyneel AV, Alain Roman, Stephane Alard, et al. (2021) post-discharge critical COVID-19 lung function related to severity of radiologic lung involvement at admission. Respir Res 22(1): 29.
- Pan F, Ye T, Sun P, Shan Gui , Bo Liang, et al. (2020) Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 295(3): 715-721.
- Han X, Fan Y, Alwalid O, Na Li, Xi Jia, et al. (2021) Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology 299: E177–E186.
- Murray MF, Kenny EE, Ritchie MD, Daniel J Rader, Allen E Bale, et al. (2020) COVID-19 outcomes and the human genome. Genet Med 22(7): 1175-1177.
- Helen Su, National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH), Bethesda, USA and Co-leader Dr Jean-Laurent Casanova, The Rockefeller University, Howard Hughes Medical Institute (HHMI), New York, USA and Necker Hospital for Sick Children & INSERM, Paris, France.
- Godlee F (2020) Editors’s Choice - Living with covid-19. BMJ 370: m3392.
- Del Rio C, Collins LF, Malani P (2020) Long-term health consequences of COVID-19. JAMA.
- Gorna R, MacDermott N, Rayner C, Margaret O’Hara,d Sophie Evans, et al. (2021) Comment: Long COVID guidelines need to reflect lived experience. The Lancet 397(10273): 455-457.
- Sudre CH, Murray B, Varsavsky T(2020) Attributes and predictors of Long-COVID: analysis of COVID cases and their symptoms collected by the Covid Symptoms Study App. medRxiv and bioRxiv.
- Cellai M, O’Keefe JB (2020) Characterization of prolonged COVID-19 symptoms and patient comorbidities in an outpatient telemedicine cohort. medRxiv and bioRxiv.
- Carvalho-Schneider C, Laurent E, Lemaignenet A, et al. (2020) Follow-up of adults with noncritical COVID-19 two months after symptom onset, Clinical Microbiology and Infection.
- Wise J (2020) Covid-19: Symptoms are common after acute phase of disease, Italian study shows. BMJ 370.
- Glöckl R, Buhr-Schinner H, Koczulla AR, R Schipmann, K Schultz, et al. (2020) Recommendations from the German Respiratory Society for Pulmonary Rehabilitation in Patients with COVID-19]. Pneumologie 74(8): 496-504.
- Phelan D, Kim JH, Chung EH (2020) A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID-19) infection. JAMA Cardiol 5(10): 1085-1086.
- Salman D, Vishnubala D, Le Feuvre P (2021) Returning to physical activity after covid-19. BMJ 8 (372): m4721.
- Tenforde MW, Kim SS, Lindsell CJ, Erica Billig Rose, Nathan I Shapiro, et al. (2020) Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network-United States, Morb Mortal Wkly Rep 69(30): 993-998.
- Williams N (2017) The Borg Rating of Perceived Exertion (RPE) scale. Occup Med 67(5): 404-405.
- Halpin, SJ, McIvor, C, Whyatt, G, Anastasia Adams, Olivia Harvey, et al. (2021) Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: A cross-sectional evaluation. J Med Virol 93(2): 1013-1022.
- Lechowicz K, Drożdżal S, Machaj F, Jakub Rosik, Bartosz Szostak, et al (2020) COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J Clin Med 9(6): 1917.
- Chen JY, Qiao K, Liu F, Bo Wu, Xin Xu, et al. (2020) Lung transplantation as therapeutic option in acute respiratory distress syndrome for COVID-19-related pulmonary fibrosis. Chin. Med J 133(12): 1390-1396
- Bharat A, Machuca TN, Querrey M, Chitaru Kurihara , Rafael Garza-Castillon, et al. (2021) Early outcomes after lung transplantation for severe COVID-19: a series of the first consecutive cases from four countries. Lancet Respir Med 9(5): 487-497.
- Nalbandian A, Sehgal K, Gupta A, Mahesh V Madhavan, Claire McGroder, et al. (2021) Post-acute COVID-19 syndrome. Nat Med 27: 601-615.

 Review Article
Review Article