Abstract
We reported the case of central nervous system relapse of acute myeloid leukemia FLT3-ITD and NPM1 positive, diagnosed and monitored over time by flow cytometry and quantitative analysis of molecular markers.
Keywords: AML; FLT3-ITD; NPM; CNS Relapse
Abbreviations: AML: Acute Myeloid Leukemia; NPM: Nucleophosmin; CSF: Cerebrospinal Fluid; LDH: High Lactate Dehydrogenase; FAB: French-American-British; ALL: Acute Lymphoblastic Leukemia
Case Report
We here describe a 58 years-old male patient diagnosed in September 2019 with acute myeloid leukemia (AML) in another hematological center and referred to us to receive allogeneic stem cell transplantation (HSCT). At the diagnosis patient showed an ECOG performance score of 2 and hyperleukocytosis (WBC 120*10^9/L). Bone marrow smear was conclusive for acute monocytic leukemia (FAB M5) and flow cytometry (MFC) identified a blasts population positive for CD33, CD13, CD64, CD14 and CD56 antigens. Karyotype analysis reported aneuploidy (35-45 chromosomes) in 11 metaphases out of 20. Next-generation sequencing analysis revealed a missense mutation in DNA methyltransferase gene 3A (DNMT3A) at exon 23, frameshift insertion in nucleophosmin1 (NPM1) gene at exon 11 and frameshift insertion in Fms-like tyrosine kinase internal tandem duplication (FLT3 ITD) gene at exon 14. The patient received induction chemotherapy according to 3+7 schedule and a consolidation cycle with intermediate dose of cytarabine (1g/m2 on day 1-3-5) combined with FLT3-inhibitor midostaurin (50mg bid from day 8 to day 21), obtaining a complete remission. Pre-transplant exams performed in March 2020 revealed an haematological relapse with a blast count of 28% in peripheral blood, WBC 4.48*10^9/L, PLTs 89*10^9/L, and Hgb 12.6g/dl. For this reason allogeneic transplantation was postponed and sorafenib off label was started at the initial dose of 200mg bid. Two weeks later, the patient came to the outpatient clinic referring neck pain and stiffness. He was promptly admitted to our transplant unit.
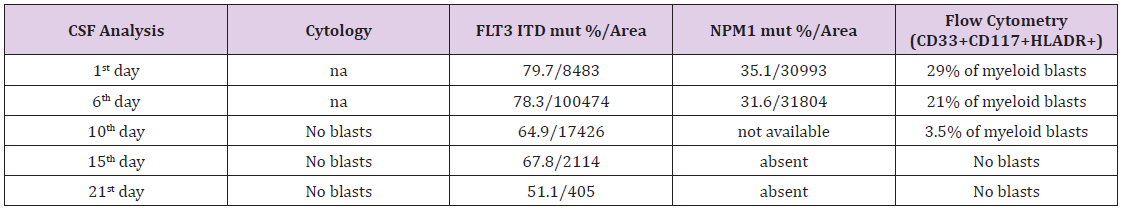
A brain CT scan appeared negative except for cervical facet arthrosis. Brain and medullary MRI showed only abnormal bone marrow signal into vertebral bodies due to leukemia replacement of bone marrow. After ophthalmological evaluation in order to exclude papillary edema, a diagnostic lumbar puncture was performed. Conventional cytology and chemistry on cerebrospinal fluid (CSF) did not revealed the presence of cells nor abnormalities in protein and glucose levels. Microbiological cultures resulted negative for cytomegalovirus, cryptococcus, fungi, syphilis, herpes viruses, polyomavirus, enterovirus and mycobacterium. Molecular exams for AML displayed a positivity for FLT3 ITD (79.7%/area 8483) and NPM1 (35.1%/area 30993). CSF immunophenotype analysis (MFC) revealed the presence of a blasts population accounting for the 29% of the total CSF cellularity, positive (high expression) for CD117, CD13, CD33, with a partial expression of CD34 antigen. Patient continued sorafenib treatment and triple intrathecal therapy (methotrexate 10mg, cytarabine 40mg and methylprednisolone 40mg) was added approximatively every 5 days, for a total of 5 procedures before transplantation (Table 1). Thrombocytopenia refrained from further intrathecal procedures. Starting from the second intrathecal procedure, patient showed improvement of symptoms and CSF clearance was monitored using both FMC and quantitative analysis of FLT ITD and NPM1 transcript (Figure 1).
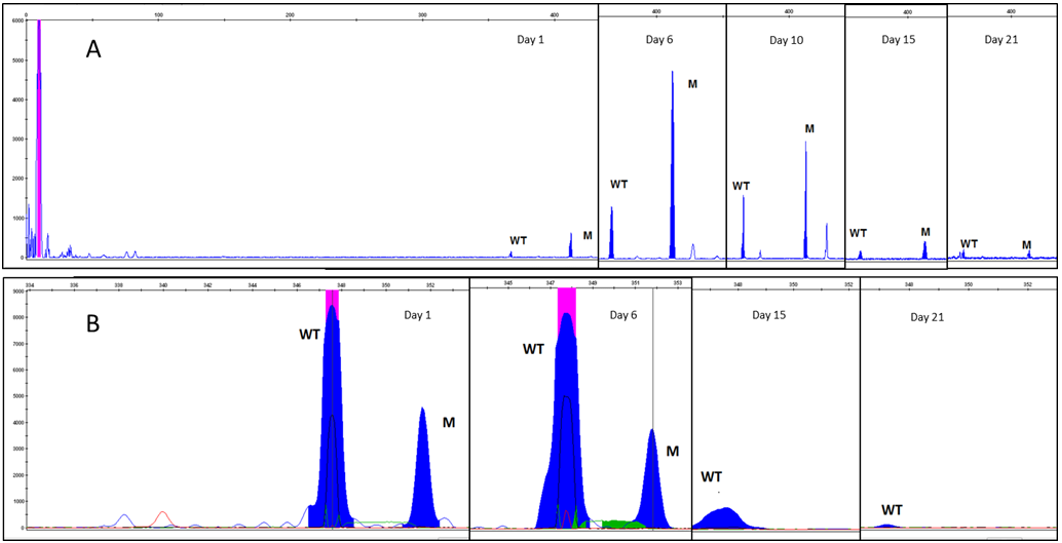
Figure 1: Quantitative molecular monitoring on cerebrospinal fluid during treatment.
A. Figure A showed FLT3-ITD peak quantification on CSF for each lumbar puncture performed.
B. FigureB showed NPM1 peak quantification on CSF for each lumbar puncture.
The last two lumbar punctures resulted to be positive only for FLT3 ITD mutation, whereas FMC was negative as well as NPM1 signal disappeared. A week after the last lumbar puncture, transplant conditioning was started as follows: thiotepa 5mg/ Kg on day -6 and -5, fludarabine 50mg/m2 from day -4 to day -2, busulphan 3.2mg/Kg on day –4 and -3. On day 0 the patient received stem cells infusion from his HLA identical sister (total CD34+ 7.95*10^6/Kg). Graft versus host disease prophylaxis was cyclosporine A 3mg/Kg bid and mycophenolic acid 15mg/Kg bid starting from day 0, and cyclophosphamide 50mg/Kg on days +3 and +5. On day +7 after transplant, patient complained of persistent headache. A CT scan showed multiple small hemorrhagic foci with mild mass effect on surrounding parenchyma and associated CSF hypotension features. The brain and medullary MRI confirmed CSF hypotension features together with multiple subdural and perispinal hemorrhagic spots and epidural vein hypertrophy. Patient received adequate fluid therapy, platelet transfusion to maintain a count above 20*10^9/L, and preferred supine decubitus with symptoms improvement. A new MRI evaluation 10 day after, showed a resolution of the previous documented bleeding, with persistence of CSF hypotension. After hematological reconstitution the patient received brain irradiation as consolidation treatment for CNS localization (total dose 18 Gy).
Central nervous system involvement in adult patients affected by AML varied from 0.6% to 10% among series [1-5] with a high incidence for patients suffering a relapse as compared to newly diagnosed AML [6-8]. Patients at high risk for developing CNS disease are those showing one of the following characteristics at the time of AML diagnosis: young age, high white blood count, 11q23 abnormalities, inv(16), +8, complex aberrant karyotype, FLT3-ITD mutation alone or combined with NPM1 mutations, French-American-British (FAB) M5 morphology, high peripheral blood blasts percentage and high lactate dehydrogenase (LDH) levels [1-4, 6,9]. Conflicting data existed about survival outcome for patients with CNS involvement. Cheng and colleagues [1] reported as a dismal outcome for patients developing late CNS relapse, whereas no reduction in survival was reported for patients with concurrent CNS involvement at AML diagnosis. Opposite to that, an overall low response rate and a worse outcome were described by Alakel [6]. Interestingly, it was reported that HSCT might confer an advantage in terms of survival for those patients who achieved a CNS remission before transplantation [9]. In this setting, high white blood count at diagnosis and residual AML in the CSF or in the bone marrow before transplantation have been reported as predisposing factors for CNS AML relapse, but the achievement of a complete remission before transplantation offered a good survival after HSCT [10]. To date there are no recommendation regarding routine intrathecal prophylaxis before and/or after HSCT for patients without prior CNS involvement in AML. However, a lumbar puncture should be performed before HSCT in patients at high risk for CNS involvement including those subjects with previous CNS disease, relapsed/refractory AML and previous extramedullary disease [5]. In our patient a concurrent medullary and CNS AML relapse was documented, both positive for FLT3 ITD and NPM1 mutations. We here highlight the role of both MFC and molecular monitoring in making a diagnosis of AML involvement in CSF. Recently the issue of the so called occult CNS leukemia with MFC has increasingly been recognized as a strong predictor for subsequent disease relapse in acute lymphoblastic leukemia (ALL) [11,12]. In this case we combined quantitative analysis of molecular markers and MCF and it was useful to identify leukemia and residual disease on CSF particularly when MCF was under the limit of detection and for monitoring response throughout the treatment. Since no specific diagnostic criteria are classified to ascertain CSF contamination in AML, combining modalities should be pursued waiting for standardization and harmonization of these procedures.
Acknowledgment
This study was supported by Centro di ricerca sulle cellule staminali emopoietiche e le terapie cellulari, Università Cattolica del Sacro Cuore, Largo Francesco Vito 1, 00168, Rome, Italy. This study was approved by the internal review board of the Hematology Institute of Fondazione Policlinico Universitario Agostino Gemelli IRCCS.
Authorship
EM and SG wrote the paper, SB performed flow cytometry analysis, VA recorded clinical data, GM and MR performed molecular tests, FS, LL and MB managed the patient during hospitalization, AB and SS punctually revised the paper, PC idealized the work and wrote the paper.
Conflict of Interest
Authors declare no competing financial interest.
References
- Cheng CL, Li CC, Hou HA, Wei Quan Fang, Chin Hao Chang, et al. (2015) Risk Factors and Clinical Outcomes of Acute Myeloid Leukaemia With Central Nervous System Involvement in Adults. BMC Cancer 15: 344.
- Rozovsky U, Ohanian M, Ravandi F, Guillermo Garcia-Manero, Stefan Faderl, et al. (2015) Incidence of and Risk Factors for Involvement of the Central Nervous System in Acute Myeloid Leukemia. Leuk Lymphoma 56(5): 1392-1397.
- Shihadeh F, Reed V, Faderl S, L Jeffrey Medeiros, Ali Mazloom, et al. (2012) Cytogenetic Profile of Patients with Acute Myeloid Leukemia and Central Nervous System Disease. Cancer 118(1): 112-117.
- Jabbour E, Daver NG, Short NJ, Xuelin Huang, Hsiang-Chun Chen, et al. (2017) Factors Associated with Risk of Central Nervous System Relapse in Patients with Non-Core Binding Factor Acute Myeloid Leukemia. Am J Hematol 92(9): 924-928.
- Bommer M, Von Harsdorf S, Döhner H, Donald Bunjes, Mark Ringhoffer (2010) Neoplastic Meningitis in Patients with Acute Myeloid Leukemia Scheduled for Allogeneic Hematopoietic Stem Cell Transplantation. Haematologica 95(11): 1969-1972.
- Alakel N, Stölzel F, Mohr B, Michael Kramer, Uta Oelschlägel, et al. (2017) Symptomatic Central Nervous System Involvement in Adult Patients with Acute Myeloid Leukemia. Cancer Manag Res 9: 97-102.
- Fianchi L, Quattrone M, Criscuolo M, Silvia Bellesi, Giulia Dragonetti, et al. (2021) Extramedullary Involvement in Acute Myeloid Leukemia. A Single Center Ten Years' Experience. Mediterr J Hematol Infect Dis 13(1): e2021030.
- Del Principe MI, Buccisano F, Soddu S, Luca Maurillo, Mariagiovanna Cefalo, et al. (2018) Involvement of central nervous system in adult patients with acute myeloid leukemia: Incidence and impact on outcome. Semin Hematol 55(4): 209-214.
- Patkowska E, Szczepaniak A, Barańska M, Maciej Kaźmierczak, Monika Paluszewska, et al. (2019) Primary and Secondary Central Nervous System Involvement in Acute Myeloid Leukemia. J Leuk 7(2): 257.
- Ikegawa S, Doki N, Kaito S, Shuhei Kurosawa, Masahiro Sakaguchi, et al. (2017) Central Nervous System Involvement at the Time of Allogeneic Hematopoietic Stem Cell Transplantation Is Associated with a Poor Outcome in Patients with Acute Myeloid Leukemia. Pathol Oncol Res 23: 433-437.
- Del Principe MI, Gatti A, Johansson U, Buccisano F, Brando B (2021) ESCCA/ISCCA protocol for the analysis of cerebrospinal fluid by multiparametric flow-cytometry in hematological malignancies. Cytometry B Clin Cytom 100(3): 269-281.
- Bento LC, Correia RP, Alexandre AM, Sonia Tsukasa Nosawa, Eduardo de Carvalho Pedro, et al. (2018) Detection of Central Nervous System Infiltration by Myeloid and Lymphoid Hematologic Neoplasms Using Flow Cytometry Analysis: Diagnostic Accuracy Study. Front Med (Lausanne) 5: 70.

 Case Report
Case Report