ABSTRACT
This study evaluated the effect of oral intake of coffee and aspartame on the oxidative status and some cytokines levels in varied body mass index Wistar rats. Ninety-six male Wistar rats were allotted into 2 sets of four groups respectively, comprised of twelve rats with average weights of 147.15 ± 4.96 g (normal body mass index (N-BMI) and 269.42 ± 6.14 g (high body mass index (H-BMI). The rats were administered coffee and aspartame such that: Groups A and B rats were respectively N-BMI and H-BMI, received distilled water; Groups C and D rats were respectively N-BMI and H-BMI, received coffee; while E and F rats were respectively N-BMI and H-BMI, received aspartame; and G and H rats were respectively N-BMI and H-BMI, received aspartame and coffee. Six rats from each group were sacrificed after 6 weeks of administration, while the remaining six rats were sacrificed after 12 weeks of administration. The concentrations of nitric oxide (NO), superoxide dismutase (SOD), reduced glutathione (GSH), malondialdehyde (MDA), catalase, protein carbonyl (PC), protein thiol (PT), S-glutathionylated protein (S-GP), C-reactive protein (CRP), interleukin 6 (IL6), interleukin 1β (IL-1β), vascular cell adhesion molecule (VCAM-1), monocyte chemotactic protein-1 (MCP-1), oxidized low density lipoprotein cholesterol (ox-LDLC), paraoxonase (PON-1) and total immunoglobulin (IGT) were determined in the serum, plasma and tissues homogenate as applicable. The histo-pathological analysis was performed on the liver. The results of the study indicated decreases (p<0.05) in NO, MDA, PC, S-GP in N-BMI rats administered coffee and aspartame both at 6 weeks and 12 weeks, while increases were obtained in GSH and SOD in the coffee administered N-BMI rats. Marked increases were recorded in NO, IL-6, IL-1β, CRP, MCP-1, VCAM-1, ox-LDLC and IGT both at week 6 and week 12 in no duration dependent manners (p<0.05) across the groups. Photomicrographs of the liver of H-BMI and aspartame N-BMI rats indicated varied alteration in the histo-architecture of hepatocytes. The present data indicated that the sole administration of coffee ameliorated some inflammatory perturbations recorded in the H-BMI rats. However, the trend was not sustained in the N-BMI and H-BMI rats that received aspartame, rather the exacerbation of the perturbations were recorded. Consequently, it is recommended that long-term consumption of aspartame might be correlated with oxidative stress and inflammatory disorders, and BMI is directly implicated in the aforementioned conditions.
Keywords: Coffee; Aspartame; Body Mass Index; Oxidative Stress; Inflammatory Disorders
Introduction
Body fatness has been an important psychosocial issue among humans since time immemorial. Traditionally, a person’s fatness can be defined at a personal level, as well as at a societal level. Thus, every individual has his / her own perception of how fat he / she could be. At the societal level, although it is poorly described or quantified, but there also is a degree of fatness beyond which a person generally is considered to be unacceptably fat; that is, there is an ill-defined threshold at which a person is labeled as being “too fat” or “obese.” The social consequences of being “obese” are severe [1]. Discrimination begins in childhood and results in serious emotional scars. Societal discrimination limits career choices, and indeed many career paths are closed to those considered to be obese. Also, societal stigmatization often impairs a person’s ability to express his/her intellectual and other talents; that is, they are often termed underachievers [2]. Clinically, obesity is an epidemic in the present world that has caused a lot of health concerns. Obesity raises the risk of early mortality and other chronic diseases and is associated with increased health care costs, placing a large financial burden on the populace [3]. In fact, it was considered that six out of 10 adults are considered to be overweight, which is determined by the body mass index. Body Mass Index (BMI) is a measurement of the weight with respect to the height of an individual. It is more of an indicator than the direct measurement of a person’s total body fat. Thus, as the BMI score increases, so does a person’s total body fat [4].
The degree of fatness has various interpretations and acceptance in different cultures is not clear. However, it may have depended on the availability of a reliable food supply and the effort required in obtaining it. This concept has moderated but still influences people’s views of being smart, healthy, mentally alert, as well as the good eating habits. All these factors have necessitated the need to control or be conscious of individual’s BMI to avoid the negative societal and health issues associated with being overweight. Plausibly, this could be the reason for the increase in the consumption more of fibers and low-calorie diets, the use of energy mobilizers, such as coffee, caffeinated drinks or chocolate, the preferred use of no calorie sweeteners instead of sugar, moderate consumption of grape wines, herbal preparations etc., so as to control body fat. Therefore, the consumption of caffeine, either in coffee or carbonated cola drinks (diet coke), as well as the use of aspartame as sweetener by individuals that seek to control the BMI can’t be overemphasized. Coffee is one of the most widely used non-alcoholic beverages in developing and developed countries. The coffee tree belongs to the Rubiaceae family, genus Coffea. About 80 coffee species have been identified worldwide, but only two are economically important [5]. The two economically important species are Coffea arabica, also known as Arabica coffee and Coffea canephora or Robusta coffee [6,7]. Although, coffee is made up of several components, caffeine is the most abundant constituent of coffee. Other common dietary sources of caffeine are tea, diet coke, cola soft drink, chocolates and so on. Caffeine exerts a variety of stimulatory effects upon the central nervous system, and it is probably the most widely used psychoactive substance. It also increases the respiratory rate, causes bronchi dilation, and stimulates lipolysis [8]. It is well documented that caffeine is a psychoactive drug associated with increased cognition and alertness. In fact, the stimulating effect of caffeine is the core pharmacological component of coffee. Thus, coffee is consumed largely by many in other to be active and it is part of everyone’s daily drink.
Aspartame, L-aspartyl-L-phenylalanine methyl ester, is a white powder with no odor that is extremely sweet to taste. It is about 200 times sweeter than sugar thus a very small amount provides the same sweet taste as sugar. Aspartame is a non-nutritive intense artificial sweetener used by the general public as an alternative to sugar [9,10]. Since regulatory approval about 20 years ago, the high-intensity sweetener, aspartame, is consumed in more than 6000 products by hundreds of millions of people around the world who want to enjoy the sweet taste of sugar without all the calories [11]. It is available in various food products such as beverages, dairy products, cereals, chewing gums, canned fruits, energy drink, zero coke, desserts, confections etc. Metabolism of aspartame generates approximately 50 % phenylalanine, 40 % aspartic acid and 10 % methanol respectively [12]. Phenylalanine and aspartic acid are both amino acids which are found in natural proteins and under normal circumstances are beneficial, if not essential, for health [13]. A small amount of aspartame can relatively increase plasma methanol levels. Methanol is being increasingly recognized as a substance that damages the liver cells where it is oxidized to formaldehyde and later to formate [14]. Although several studies have reported the individual effects of coffee and aspartame on the health statuses [15-20], but no literatures are available on caffeine or coffee consumption in combination with aspartame on the different biochemical variables in individuals with either high BMI or normal BMI. Relatively, there is dearth of information on the relationship between the BMI and the use of caffeine or coffee, and aspartame coadministration as found in diet coke, energy drinks etc. Hence, this study was undertaken to evaluate some health implications of the consumption of coffee and aspartame as a sweetener in normal and overweight consumers. We, therefore, investigated the oxidative status and patterns in some immunological proteins following the sub-chronic administration of coffee and aspartame singly or in combination, in normal and overweight male Wistar rats.
Materials and Methods
Materials
Experimental Animals: Ninety-six apparently healthy male Wistar rats with weights of 110 - 138 g were obtained from the animal house facility, Ladoke Akintola University of Technology, Ogbomoso, Oyo State, Nigeria.
Coffee and Aspartame: Coffee was a product of Nestle Plc. Ilupeju Lagos, Nigeria. Aspartame was a product of Nutri Limited Herbstone. U.S.A.
Reagents Kits: Quantitative assay kits of superoxide dismutase, glutathione peroxidase, malon-dialdehyde are products of Fortress Diagnostic Laboratory. The assay kits for interleukin-6, interleukin- 1β, C-reactive protein, nitric oxide and monocyte chemotactic protein-1 are products of RayBio Technology, inc. U.S.A. The assay kit for S-glutathionylated protein is a product of Cayman chemical, 1180 E Ellsworth Road, Ann Arbor MI- U.S.A.The analytical kit for oxidized low-density lipoprotein cholesterol is a product of Cusabio Biotech Co limited, www.cusabio.cn. The assay kit for vascular cell adhesion molecule is a product of Cloud Clone Corporation, 1304 Langham Creek Dr, Suite 164, Houston, Texas 77084 U.S.A. All reagents were of analytical grade.
Methods
Animal Handling, Administration of Coffee and Aspartame
The rats were kept in compartmentalized cages and grouped into eight, comprising of twelve animals each. They were allowed access to standard pellets and distilled water. The cages were cleaned every day and the animals were kept under condition of uniform humidity and temperature on a 12-hour light - dark cycle. The animals were allowed to acclimatize for two weeks, during which they were weighed and properly observed for any changes in their behavior. Of the ninety-six rats, forty-eight were fed standard rat pellet, while the other forty eight were fed atherogenic diet for the period of six weeks to achieve mean weights of 147.15 ± 4.96 g (normal body mass index (N-BMI) and 269.42 ± 6.14 g (high body mass index (H-BMI). respectively. Coffee and aspartame were administered orally at 35 and 60 mg/kg body weight respectively to the rats such that: Groups A and B rats respectively were N-BMI and H-BMI and received distilled water. Groups C and D rats were respectively N-BMI and H-BMI and received coffee, while E and F rats were respectively N-BMI and H-BMI and received aspartame. Rats in groups G and H were respectively N-BMI and H-BMI and received aspartame and coffee concurrently. After 6 weeks of administration, six rats from each group were sacrificed, while the remaining six rats were sacrificed after 12 weeks of administration. The feed intake and body weights of the rats were monitored during the administration.
Collection of Plasma and Serum
The rats were fasted overnight and anaesthetized using diethyl ether. Blood was collected via cardiac puncture and the plasma and serum were prepared for each drawn blood. Organs of interest, such as the liver and brain were harvested immediately, cleansed of blood and rinsed with normal saline solution and the weight recorded. The organs were minced, and a small part was fixed in 10 % formasaline for histopathology examination.
Determination of Biochemical Parameters
Oxidative Status: The activities of superoxide dismutase and catalase were determined as instructed in the reagent kit manuals, as well as the concentrations of reduced glutathione, malondialdehyde, nitric oxide, protein carbonyl and total protein thiol serum or plasma and tissues homogenate as applicable.
Immunological Proteins: The concentrations S-glutathionylated protein, oxidized low density lipoprotein cholesterol, C-reactive protein, interleukin 1β, interleukin 6, vascular cell adhesion molecule and monocyte chemotactic protein- 1β were determined in the serum or plasma as instructed in the ELISA kit manual.
Total Immunoglobulin: Total immunoglobulin concentration was carried out according to the method described by Pfeiffer et al. (1977). The method is a semi-quantitative test for plasma globulin, and it was based on precipitation of globulin by low concentrations of metal ions and subsequent turbidometric analysis.
Histopathology: Histological procedures were performed in the liver according to the method of Avwioro, 2010.
Statistical Analysis: This research work was a completely randomized design (CRD), and the results were expressed as mean of at least four replicates ± standard error of mean (SEM). Data were subjected to one way analysis of variance (ANOVA), after which the Duncan Multiple Range Test (DMRT) was conducted for the pair-wise mean comparison and determination of the variation within the groups. Values were considered statistically significant at (p<0.05) and denoted by alphabets.
Results
Oxidative Stress Indices
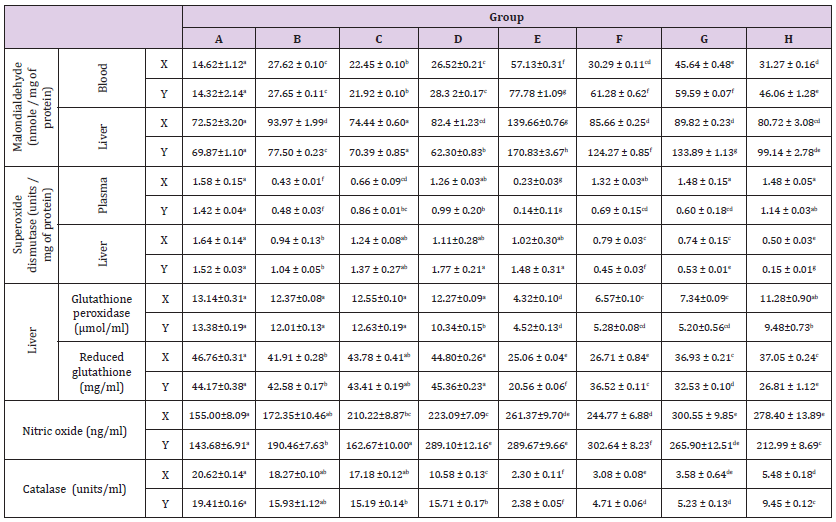
The trends obtained in the oxidative stress indices determined in this study were presented in Table 1. Marked reductions (p<0.05) were recorded in the concentration of reduced glutathione in the liver of rats across the groups in a manner not dependent on the duration of administration, except in groups C and D rats (p>0.05). Glutathione peroxidase concentrations were not altered (p<0.05) in the livers of rats in groups B and C at both weeks, and groups D and Hat six weeks only. The production of nitric oxide in the plasma and malondialdehyde concentrations in the liver of rats increased (p<0.05) across the groups in a duration dependent manner, except in rats in group B, while malondialdehyde concentrations in the blood increased (p<0.05) consistently across all the groups Table 1. The activities of superoxide dismutase decreased (p<0.05) in the liver and plasma of the rats across the groups, which were dependent on the duration. In a similar manner, catalase activities in the liver were diminished across the groups (p<0.05), except in group B (p>0.05).
Table 1: Trends of some oxidative stress indices in male Wistar rats administered with coffee and aspartame.
Note: A-Control N-BMI, B-Control H-BMI, C-Coffee N-BMI, D- Coffee H-BMI, E-Aspartame N-BMI, F-Aspartame H-BMI, G-Aspartame + coffee N-BMI, H-Aspartame + Coffee H-BMI. N-BMI = Normal body mass index and H-BMI = High body mass index. X denotes 6weeks, Y denotes 12 weeks. Values are means ± SEM, mean values bearing different alphabets are significantly different (P<0.05).
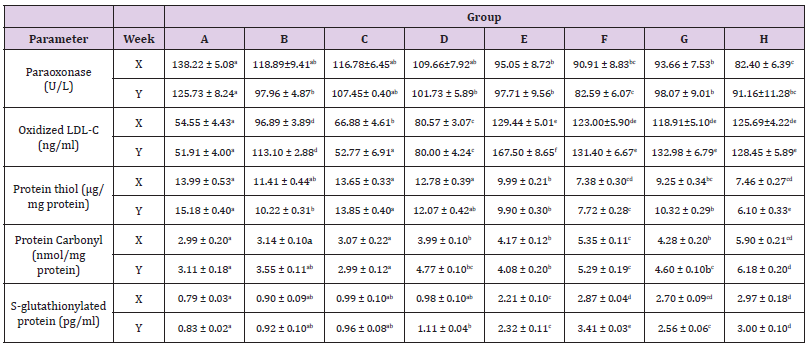
Table 2: Concentrations of some inflammatory indices in male Wistar rats administered coffee and aspartame.
Note: A-Control N-BMI, B-Control H-BMI, C-Coffee N-BMI, D- Coffee H-BMI, E-Aspartame N-BMI, F-Aspartame H-BMI, G-Aspartame + coffee N-BMI, H-Aspartame + Coffee H-BMI. N-BMI = Normal body mass index and H-BMI = High body mass index X denotes 6weeks, Y denotes 12 weeks. Values are means ± SEM, mean values bearing different alphabets are significantly different (P<0.05).
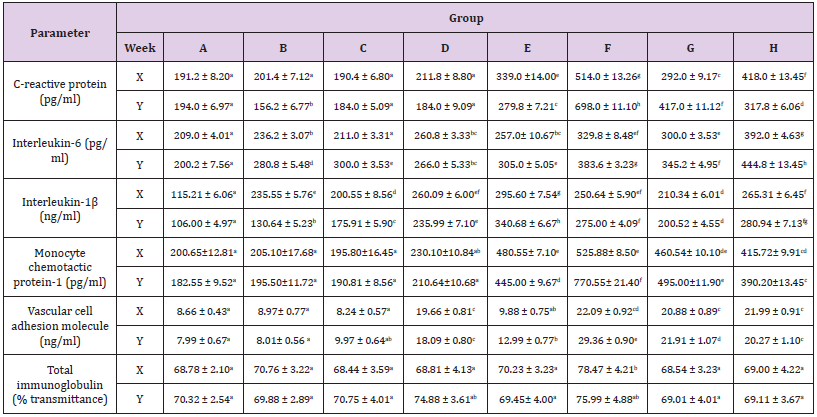
Table 3: Concentrations of some cytokines and total immunoglobulin in Wistar rats administered coffee and aspartame.
Note: A=Control N-BMI, B=Control H-BMI, C=Coffee N-BMI, D=Coffee H-BMI, E=Aspartame N-BMI, F=Aspartame H-BMI, G=Aspartame + coffee N-BMI, H=Aspartame + Coffee H-BMI. N-BMI = Normal body mass index and H-BMI = High body mass index, X denotes 6weeks and Y denotes 12 weeks. Values are means ± SEM, mean values bearing different alphabets are significantly different (P<0.05)
Inflammatory Indices
The results obtained in the inflammatory indices determined in this study were depicted in the Table 2. The activities of paraoxonase (PON-1) decreased (p<0.05) across the groups of rats with high body mass indexes and aspartame (groups B & D – H). Oxidized low density lipoprotein cholesterol concentrations were decreased (p<0.05) across the groups in a trend dependent on the duration Table 2, except in group C (p>0.05). The concentrations of protein thiol decreased (p<0.05) in rats administered aspartame, except in groups C and D at both weeks, and group B at 6 weeks (p>0.05). Protein carbonyl and S-glutathionylated protein concentrations increased (p<0.05) in groups E - H at both weeks.
Immunological Parameters
Depicted in Table 3 were the patterns recorded in the immunological parameters assayed in our study. The concentration of C-reactive protein increased (p<0.05) in all the rats administered with aspartame (groups E - H) at both weeks but decreased (p<0.05) at six weeks in the rats that received coffee only (groups C and D). Interleukins -1β and -6 concentrations increased (p<0.05) across the groups in a somewhat duration dependent with no alteration (p<0.05) in interleukin-6 concentration in the coffee only administered rat (group C) at six weeks Table 3. Increases were obtained in the vascular cell adhesion molecule and monocyte chemotactic protein-1 (p<0.05) in rats administered aspartame (groups E – H). The total immunoglobulin concentrations were relatively unaltered (p>0.05) across the groups, except in rats in group F that indicated increases (p<0.05) at 6 weeks.
Histopathology
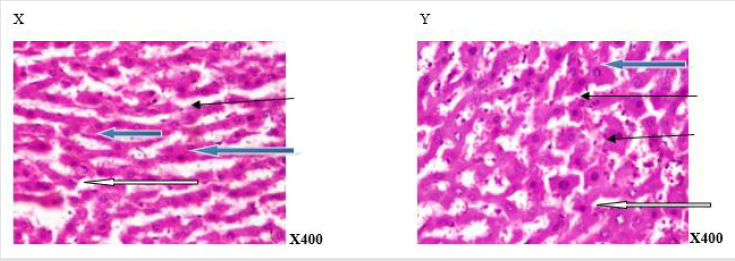
Plates 1 - 8 depicted the photomicrographs of the liver sections of rats administered aspartame, and coffee. The photomicrograph of the liver section of the control rat (group A) displayed normal central venule, sinunoids and cytomorphorlogy (Plate 1). Plates 2 and 4 indicated hepatocytes with varied percentage of normal central venules and cytomorphology with mildly infiltrated sinusoids at 6 weeks and mildly - moderately infiltrated sinusoids and or, mild portal triad with bile duct hyperplasia at 12 weeks. In Plate 3, the photomicrograph indicated normal central venule and cytomorphology, and sinusoids with very mild infiltration of inflammatory cells at 6 weeks and mild perivascular infiltration at 12 weeks. The photomicrographs in plates 5 and 7 showed normal central venule and normal architecture with mild dilation of the sinusoids by infiltration of inflammatory cells at 6 weeks, but oedema and necrosis were seen in the sinusoid moderately at 12 weeks. In Plate 6, the photomicrographs of the liver revealed varied percentage of central venules with mild congestion, mild portal triditis and cytoplasmic vacuolation and lysed cells in the sinusoids at weeks 6 and 12 respectively. The photomicrographs in Plate 8 indicated mild - poor cytomorphology with vesicular nuclei, normal - mildly congested venule and lymphocytes aggregation, and hyperchromic nuclei in the sinusoids at 6 and 12 weeks respectively.
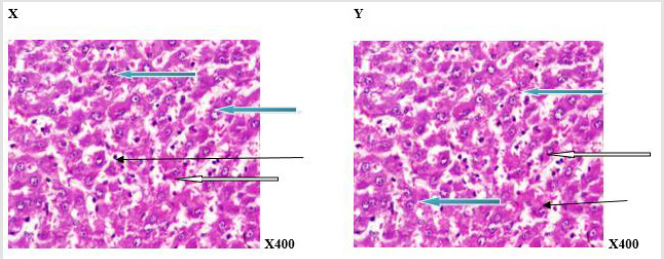
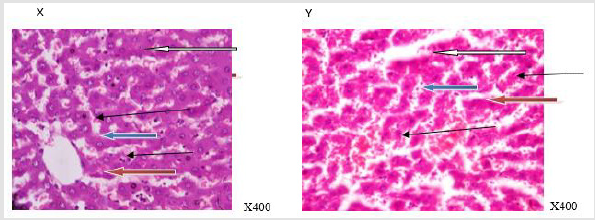
Figure 1:Photomicrograph of a liver section of rat adminitered distilled water (N-BMI). X and Y showed normal central venule (white arrow) and moderately normal architecture. The sinusoid appear normal(slender arrow), the hepatocytes show normal cytomorphology (blue arrow) X= 6 weeks and Y= 12 weeks.
Figure 2:Photomicrograph of a liver section of rats adminitered distilled water (H-BMI). X and Y showed portal triad with bile duct hyperplasia (white arrow) and fat deposits (black arrow) at the perpheral of the portal vein. The sinusoid shows mild infiltration of inflammatory cells (slender arrow). Hepatocytes show normal morphology (blue arrow) X=6 weeks and Y = 12 weeks.
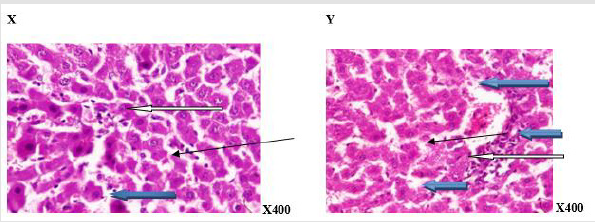
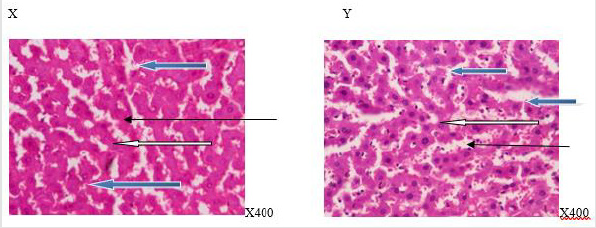
Figure 3:Photomicrograph of a liver section of rats given coffee (N-BMI). X showed normal central venule (white arrow), the sinusoid showed very mild infiltration of inflammatory cells (slender arrow), some of the hepatocytes show normal cytomorphology (blue arrow). In, Y, there is mild perivascular infiltration (white arrow), the sinusoid showed no infiltration of inflammatory cells and appears normal (slender arrow), the hepatocytes appear normal (blue arrow). X=6 week and Y= 12 week.
Figure 4:Photomicrograph of a liver section of rats given coffee (H-BMI). X showed normal central venule (white arrow), moderately normal architecture, the sinusoid appear normal (slender arrow), the hepatocytes show normal cytomorphology (blue arrow). Y showed moderate architecture, there is mild perivascular infiltration (white arrow) the sinusoid show mild dilation with very mild infiltration of inflammatory cells. (Slender arrow), the hepatocytes appear normal (blue arrow).X= 6 week and Y=12week.
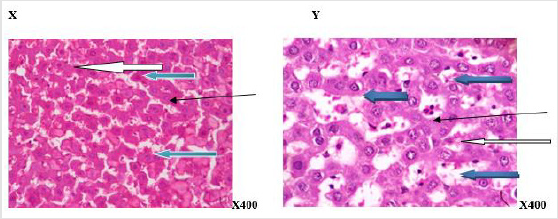
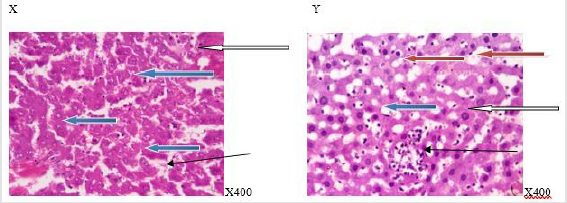
Figure 5:Photomicrograph of a liver section of rats given aspartame (N-BMI). X showed normal central venule (white arrow) and moderately normal architecture, the sinusoid show very mild infiltration of inflammatory cells (slender arrow), the hepatocytes show normal cytomorphology (blue arrow). Y showed moderate to severe perivascular infiltration (white arrow). There is an area of edema noted (black arrow), the sinusoid appear mildly dilated with infiltration of inflammatory cells (slender arrow), the hepatocytes show cytomorphology with necrosis (blue arrow). X=6 weeks and Y=12weeks.
Figure 6:Photomicrograph of a liver section of rats given aspartame (H-BMI). X showed normal central venule (white arrow), normal architecture, the sinusoid appear normal without infiltration of inflammatory cells (slender arrow), the hepatocytes show normal cytomorphology (blue arrow). Y showed central venule with mild congestion (white arrow) and the portal tract show mild portal triditis (black arrow, the sinusoid show attendance of lysed red cells and inflammatory cells (slender arrow), the hepatocytes show several normal (blue arrow) and few cytoplasmic vacuolation (red arrow). X=6 weeks and Y=12 weeks
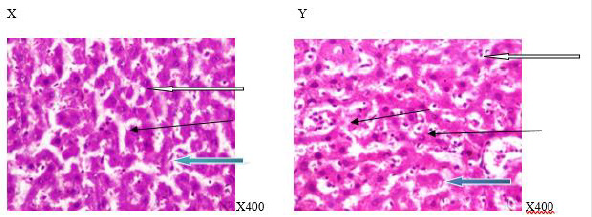
Figure 7:Photomicrograph of a liver section of rats given aspartame and coffee (N-BMI). X showed normal portal tract (white arrow) and normal architecture,the sinusoid appear normal (slender arrow), the hepatocytes show normal cytomorphology (blue arrow). Y showed moderately normal architecture (white arrow), the sinusoid appear mildly infiltrated by red cells and inflammatory cells (slender arrow) and the hepatocytes show normal cytomorphology (blue arrow). X=6weeks and Y=12weeks.
Figure 8: Photomicrograph of a liver section of rats given aspartame and coffee (H-BMI). X showed normal central venule (white arrow), moderately normal architecture, the sinusoid appear normal (slender arrow), the hepatocytes show poor cytomorphology with vesicular nuclei (blue arrow). Y showed normal central venule (white arrow) and mildly congested venule (black arrow), the sinusoid show mild mild infiltration of inflammatory cells and lymphocytes aggregate (slender arrow), some of the the hepatocytes show vesicular nuclei (red arrow) and some with hyperchromic nuclei (blue arrow) . X=6 weeks and Y = 12 weeks.
Discussion
Nitric oxide is important for the relaxation of smooth muscles to enhance the flow of blood and increase general cell health. The biological activity of nitric oxide may be modified by oxygenderived reactive species, such as anion superoxide (O2•), hydrogen peroxide (H2O2) and hydroxyl radical (OH•), contributing to regulate the vascular tone [22]. In this study, the increases recorded in the production of nitric oxide (NO•) in the rats with normal- and high- body mass index (N-BMI and H-BMI) administered with aspartame and coffee, either singly or in combination indicated predisposition to the generation of free radicals Table 1. Although, the generation of free radicals via nitric oxide can be advantageous or disadvantageous, depending on the regulation of the production and the effectiveness of the oxidant/antioxidant balance systems. NO• possesses both cytoprotective and cytotoxic properties, depending on the amount and the isoform of NOS [23]. That is, if a cell is in a pro-oxidant state, NO• can undergo oxidative-reductive reactions to form toxic compounds (these belong to a family known as ‘reactive nitrogen species, or RNS), which cause cellular damage. Scavenging or neutralization of the free radicals generated during cellular metabolism necessitates the increase in the activities of both enzymatic and non-enzymatic antioxidative systems in order to prevent oxidative cell damage [24].
The trend reported in the concentration of reduced glutathione (GSH) in the liver of rats, especially those administered with aspartame indicated the generation of free radicals, which was strengthened by the H-BMI. GSH has a multifactorial role in antioxidant defense; it is a direct scavenger of free radicals, by neutralizing hydrogen peroxide (H2O2) or indirectly by repairing initial damage to macromolecules inflicted by H2O2, as well as a co-substrate for peroxide detoxification by glutathione peroxides [25]. The patterns in the glutathione peroxidase activities in the liver of rat supported the reported depletion of GSH in the liver Table 1. Glutathione peroxidase is a major enzyme that in involved in the neutralization of free radicals generated during cellular and or, xenobiotic metabolism, and its activity is dependent on the concentration of GSH. The reductions in GSH and glutathione peroxidase indicated a burden / an overwhelming effect on the endogenous antioxidant systems. This is supported by the reported patterns in the superoxide dismutase (SOD) and catalase (CAT) activities in the livers of rats, which were amplified by the duration of administration and the BMI. In addition, the reductions in antioxidant indices (GSH, glutathione peroxidase, CAT and SOD) in the liver of rats administered aspartame and the increases when co administered with coffee, implied that coffee had ameliorative capacity on the antioxidant systems Table 1. The result of this study underlined the antioxidant activity of coffee and oxidative capabilities of aspartame. An earlier report confirmed that methanol administration could decrease the enzymatic antioxidants (SOD and CAT) in the lymphoid organs [26].
The decline in the activities of these enzymes might be due to their inactivation caused by excess reactive oxygen species (ROS) production [27]. Normally, the antioxidant enzyme, catalase protects SOD against inactivation by H2O2. Reciprocally, SOD protects catalase against superoxide anion. However, overload of free radical could upset these regulations. Furthermore, the decrease in SOD and CAT activities might be due to the formation of formaldehyde from the methanol. Previous studies have showed that aspartame metabolism produces methanol, which is oxidized to formaldehyde and in turn increase free radical production that could lead to decreases in SOD and CAT activities in the liver [28]. Malon dialdehyde (MDA) is the stable product from the oxidative degradation of polyunsaturated fatty acids. MDA can interact with proteins and is itself potentially atherogenic. That is, it can react with the free amino group of proteins, phospholipids and nucleic acids leading to structural modification, which can induce dysfunction of immune systems, which are usually correlated with the pathogenesis of various diseases such as tissue damage, atherosclerosis, stroke, cancer and Graves’ disease etc [29]. The measurement of MDA is one of the most often used biomarker to investigate the oxidative damage on lipids, the major lipid peroxidation product. The reported increases in the concentrations of MDA in the liver and blood in our study supported the indicated induction of free radicals, and the activation and depletion of the endogenous antioxidant systems Table 1. Interestingly, the concentrations MDA in the livers of rats with N-BMI and H-BMI either administered with coffee singly were more favourable than those of N-BMI and H-BMI co-administered coffee and aspartame. Although, the result obtained for concentration of MDA in the blood all depicted oxidative damage Table 1. It could be possible that the major site of oxidative damage of the metabolites of coffee was most likely not the liver. Perhaps, the blood cells or other tissues might be the preferential target sites that the metabolites of aspartame and coffee elicit more cytotoxic effects.
Paradoxically, the administration of coffee, either singly or combined with aspartame elicited some remarkable amelioration of the antioxidant capabilities in the liver of the rats, especially those rats with N-BMI. This was not to be in the plasma, as the antioxidant capabilities was weakened, as depicted in the reductions in the activities of SOD and increases in NO•, and MDA concentrations Table 1 that supported the induction of free radicals following the administration of coffee and aspartame. Thus, the indicated antioxidative capabilities of coffee and the oxidative capabilities of aspartame were strongly re-underlined.
The results obtained in the anti-inflammatory parameter, paraoxonase (PON-1) activities in the liver indicated the activation of oxidative response Table 2. PON-1 is a hydrolytic enzyme that protects against lipid peroxidation. It is primarily expressed in the liver and responsible for the antioxidative functions of high-density lipoprotein cholesterol (HDL-C). Paraoxonase in HDL-C prevent the oxidation of cholesterol in low density lipoprotein cholesterol (LDL-C) by prolonging oxidation lag phase and reducing peroxides and aldehydes contents in HDL-C. Originally, PON are enzymes known to hydrolyse exogenous toxic organophosphate compounds, but later found to possess also, arylesterase and latonase activities [30]. The reported increased concentrations of oxidized low density lipoprotein cholesterol (ox-LDL-C) in the liver of rats supported the trend obtained in the PON-1 activities Table 2.
Oxidized low density lipoprotein cholesterol contains unoxidized and oxidized fatty acid derivatives both in the ester and free forms, their decomposition products, cholesterol and its oxidized products, proteins with oxidized amino acids and cross-links, and polypeptides with varying extents of covalent modification with lipid oxidation products, and many others [31]. Although, there were no scientific associations between ox-LDL-C concentrations and the severity of inflammation, as the risk of ox-LDL-C formation did not change after adjustment of some inflammatory markers (Wu, 2006). However, oxidative modification of LDL-C promoted fatty- streak formation, the early lesion of atherosclerosis. This is because ox-LDL-C was shown to induce production of monocyte chemotactic protein-1 (MCP-1) and monocyte-colony stimulating factor and lead to inflammatory response in vascular cells [32]. The reported decreases in the concentrations of protein thiol and increases in protein carbonyl and S-glutathionylated protein in the liver in rats administered coffee and aspartame Table 2 indicated that aspartame had oxidative capabilities and coffee had sparing or little ameliorative potential when co-administered at the doses. S-glutathionylation is the reversible formation of protein mixed disulfides between cysteine residues and GSH (post-translational modification that is important to many biological processes). It is also an important form of post-translational modification that occurs during oxidative stress as well as under normal physiological conditions. Reversible protein thiol oxidation has been demonstrated in proteins with range of functions including signal transduction, ion transport, contractility and protein metabolism [33]. The sulfhydryl groups in protein thiol are very susceptible to oxidative damage and are often low in-patient suffering from disease and infection.
Protein carbonyl content in blood and tissues is a reliable indicator of protein oxidation [34]. The trend obtained in protein carbonyl was consistent with increases observed in the MDA level of aspartame administered rats and H-BMI rats (Table 1 and 2 respectively). In addition, H-BMI could have predisposed the rats to oxidative modifications that were escalated by the administration of aspartame with a weak ameliorative effect of the coffee administration. These results supported the trends reported in the oxidative indices and might indicate a reduction the quality of life. The reduction in the quality of life is plausible since the oxidation of cell membranes increases the polarity of lipid-phase surface charge and the formation of protein oligomers increase, while the number of thiol (SH) groups, molecular mobility of lipids and resistance to thermo-denaturation decreases. Recent findings have emphasized the importance of lipid peroxidation in relation to the role of caloric restriction and the extension of longevity. Long-lived mammals and birds possess low degrees of unsaturation in their cellular membranes; this led to lower levels of lipid peroxidation and lipoxidation-derived protein modification in long-lived species [35,36].
The trends we obtained in the MCP-1 concentration supported the submission of Oiwei (2014) on the effects of the concentration ox-LDL-C on Monocyte chemotactic protein-1. Monocyte chemotactic protein-1 (MCP-1) is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages. Both MCP-1 and its receptor CCR2 have been demonstrated to be induced and involved in various diseases [37]. Migration of monocytes from the blood stream across the vascular endothelium is required for routine immunological surveillance of tissues, as well as in response to inflammation. Therefore, the recorded increases in the concentrations of interleukin-1β (IL-1β) and vascular cell adhesion molecule (VCAM) in this study Table 3 gave credence to the increased need of monocytes migration from the blood stream across the vascular endothelium to support macrophage pick up of ox-LDL-C. VCAM-1 is an endothelial molecule that is expressed on endothelial cells during inflammatory diseases, while IL-1β is the first chemokine produced at the onset of inflammatory response to enhance chemotaxis of immune cells [38]. Interleukin 6 (IL-6) was primarily supposed to have interferon like activity and was named as B cell differentiation factor, but further studies showed its role as a multifunctional cytokine that regulates immune responses, the acute-phase response of the liver, hematopoiesis, and inflammation. One of the essential roles of IL-6 is the promotion of inflammatory reactions through the expansion and activation of T cells, differentiation of B cells, and the induction of acute-phase proteins by hepatocytes [39].
The trends obtained in the serum IL-6 concentration in our study is more likely to be proinflammatory, as other inflammatory markers were not overtly increased to connote massive inflammatory response Table 3. This is because IL-6 can act as proinflammatory and antiinflammatory molecule, depending on the concentrations of other inflammatory markers. At very high body temperatures, fibrinogens and other acute phase proteins, IL-6 act as an anti-inflammatory protein to regulate the activities of other inflammatory protein like tumor necrosis factor etc. This also supported the trends we obtained in the oxidative indices in the livers Table 2, as the main cellular targets of IL-6 are hepatocytes, leukocytes, T cells, B cells, and hematopoietic cells [40]. C-reactive protein (CRP) is an acute-phase protein that serves as an early marker of inflammation or infection. CRP binds to phosphocholine expressed on the surface of damaged cells, as well as to polysaccharides and peptosaccharides present on bacteria, parasites and fungi [41]. This binding activates the classical complement cascade of the immune system and modulates the activity of phagocytic cells, supporting the role of CRP in the opsonization (i.e. the process by which a pathogen is marked for ingestion and destruction by a phagocyte) of infectious agents and dead or dying cells. When the inflammation or tissue destruction is resolved, CRP levels fall, making it a useful marker for monitoring disease activity. The patterns we reported in the CRP concentrations in the rats administered aspartame Table 3 supported the oxidative cytotoxicity of aspartame and the antioxidative capabilities of coffee Table 3. In addition, the trend across the groups gave further credence to our finding that the metabolites of coffee might not target primarily the liver, but other tissues in the rats, while those of aspartame might be the liver.
The zinc sulfate turbidity (ZST) test is used to estimate the concentration of serum gamma globulin in a process called turbidimetric [39,40]. The reported concentrations of total immunoglobulins (IGT) in this study Table 3 indicated that there was no massive increase in secretions of immunoglobulins, thus, there was no serious cellular damage or infection, neither was there massive destruction/utilization of immunoglobulins, especially in the coffee administered rats. It can also be inferred that the hepatocytes were preserved as depicted in the coffee administered rats. This is logical because gamma-globulin levels are known to rise in infection, liver damage and or, liver disease, which often result in abnormal zinc sulfate turbidity in most chronic cases [41]. However, the increase in IGT in the H-BMI rat administered aspartame supported the oxidative-cytotoxicities of the metabolites of aspartame. The photomicrographs of the sections of the livers in rats administered coffee and aspartame (Plates 1 – 8), revealed that the rats with N-BMI had normal hepatocytes and preserved histo-architecture, while those with H-BMI had mild-moderate infiltration by immune cells, blood congestion with mild derangement in the integrity of the hepatocytes and were aggravated by the administration of aspartame. The overall revelations from the photomicrographs of the liver supported the results of the oxidative and inflammatory indices, as well as that of some cytokines that coffee administration was not toxic to the liver and had antioxidative capabilities. In the same vein, aspartame administration revealed cellular cytotoxicities, whose manifestations were aggravated with H-BMI.
Conclusion
The foregoing inferred that coffee had antioxidative capabilities that mitigated the oxidative cytotoxicity of aspartame and subjects with high body mass index had more risk consuming aspartame as an alternative sweetener. The long-term consumption of aspartame was unhealthy even in normal body mass index subjects; thus, frequent consumption of aspartame was not recommended.
Ethical Approval and Consent to Participate
This study was carried out in accordance with ethical laws on animal handling.
Funding
We did not receive any grant for the conduct of the study.
Authors’ Contributions
EBO conceived, designed and supervised the study, and drafted the manuscript. OJB, AEA and AJO provided the materials used in the study, collated literatures and performed the experimental procedures. IMO provided some reagents. AGE did the statistical analyses and drafted the manuscript. AAS co-supervised the study and read the final manuscript. All authors financed of the study. All authors read and approved the final manuscript with the order of author’s names.
Acknowledgements
We acknowledge the immense efforts and laboratory assistance of Dr. R. A. Ajani (LAUTECH) and the technical inputs of Messr Olabanji Debo of Bridge Scientifik Limited.
Consent for Publication
All the authors gave their consents for the publication of the study.
Competing Interests
There was no competing interest among the authors.
References
- Adelheid W, Andrey VK (2015) Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules Journal 5(2): 472-484.
- Ascherio A, Zhang SM, Hernan MA (2001) Prospective Study of Caffeine Consumption and Risk of Parkinson’s Disease in Men and Women. Annual Neurology Journal 50(1): 56-63.
- Avwioro OG (2001) Histochemistry and Tissue Pathology: Principle and Techniques. Claverianum Journal 15(8): 45-77.
- Barreiro E, Hussain S (2010) Protein Carbonylation in Skeletal Muscles: Impact on Function. Antioxidant Redox Signal 12(3): 417-429.
- Choudhary AK, Devi RS (2014) Imbalance of the Oxidant-Antioxidant Status by Aspartame in the Organs of Immune System of Wistar Albino Mice. African Journal of Pharmacy and Pharmacology 8(8): 220-230.
- Clark I, Landolt HP (2017) Coffee, Caffeine and Sleep: A Systematic Review of Epidemiological Studies and Randomized Controlled Trials. Sleep Medicine Review Journal 31(8): 70-78.
- Daniel D (2016) The Comforts of Coffee: The Role of the Coffee Ceremony in Ethiopians' Efforts to Cope with Social Upheaval during the Derg Regime. Unpublished M.Sc Thesis, Carleton University, Ottawa, pp.1974-1991.
- Darren T, Mark BP, Steve PW (2000) The Physiological Structure of Human C-Reactive Protein and its Complex with Phosphocholine. The American Journal of Human Genetics 7(2): 169-177.
- DeMejia EG, Ramirez-mares MV (2014) The Impact of Caffeine and Coffee on our Health. Trends in Endocrinology and Metabolism 25(10): 489-492.
- Echeverri D, Pizano A, Montes FR, Forcada P (2017) Acute Effect of Coffee Consumption on Arterial Stiffness, evaluated using an Oscillometric Method. Artery Research 17(6): 16-32.
- Edwin H, Keyvan KG, Chia CM, Ravibhind GM, Gemma AF, et al. (2013) Biological Markers of Oxidative Stress: Applications to Cardiovascular research and practice. Redox Biology 8(1): 483-491.
- Gan Y, Wu J, Zhang S, Li L, Cao S, et al. (2017) Association of Coffee Consumption with Risk of Colorectal Cancer: A Meta-Analysis of Prospective Cohort Studies. Oncotarget 8(12): 18699-18790.
- Gombos K, Varjas T, Orsos Z, Polyak E, Peredi J, et al. (2007) The Effect of Aspartame Administration on Oncogene and Suppressor Gene Expressions. In vivo 21(1): 89-92.
- Gunther K, Stefan S, Hanswalter Z, Heiner D (2000) The Mechanism of the Antiherpetic Activity of Zinc Sulphate. Journal of General Virology 71(12): 2989-2997.
- Hogan IM, Doherty J, Fagan E, Kennedy M, Conneely B, et al. (2016) Optimisation of the Zinc Sulphate Turbidity Test for the Determination of Immune Status. Veterinary Record 178(7): 152-169.
- Ighalo EE, Erilibe JE, Ehimigbai AR, Ezeuko VC, et al. (2015) Protective Properties of Yoyo Cleanser Bitters against Mercury II Chloride (HgCl2)- Induced Kidney Damage in Adult Wistar Rats. Journal of Biomedical Sciences, 14 (2): 118-128.
- Iman MM (2011) Effect of Aspartame on some Oxidative Stress Parameters in Liver and Kidney of Rats. African Journal of Pharmacy and Pharmacology 5(6): 678-682.
- Iwai K, Kishimoto N, Kakino Y (2004) In Vitro Antioxidative Effects and Tyrosinase Inhibitory Activities of Seven Hydroxycinnamoyl Derivatives in Green Coffee Beans. Journal of Agriculture and Food Chemistry 52(13): 4893-4898.
- Joan Ml, Cook M, Michelle E (2011) Vascular Cell Adhesion Molecule 1 Expression and Signaling During Disease Regulation by Reactive Oxygen Species and Antioxidants. Antioxidants and Redox Signaling Journal 15(2): 3522-3541.
- Kamel EA (2015) Inflammation and Neurochemical Alterations Induced by Oral Aspartame Administration in Experimental Rats. Indian Journal of Applied Research 5(10): 750-753.
- Kurobe K, Nakao S, Nishiwaki M, Matsumoto N (2017) Combined Effect of Coffee Ingestion and Repeated Bouts of Low‐Intensity Exercise on Fat Oxidation. Clinical Physiology and Functional Imaging 37(2): 148-154.
- Litvinov D, Mahini H, Garelnabi M (2012) Antioxidant and antiinflammmatory role of paraoxonase 1: implicationin artherosclerosis diseases. North Amer J of Med Scie 4(11): 523-532.
- Lius S, Lezanne O, Scott R (2016) Nitric Oxide: A Regulator of Cellular Function in Health and Disease. Oxidative Medicine and Cellular Longetitity 9(6): 823-846.
- Lopez GE, Rodriguez AF, Rexrode KM, Logroscino G, Hu FB, et al. (2009) Coffee Consumption and Risk of Stroke in Women. Circulation Research 119(8): 1116-1123.
- Mahboob M, Shireen KF, Atkinson A, Khan AT (2001) Lipid Peroxidation and Antioxidant Enzyme Activity in Different Organs of Mice Exposed to Low Level of Mercury. Journal of Environmental Science, 36(5): 687-697.
- Mantovani A, Garlanda C, Doni A, Bottazi B (2008) Pentraxins in innate immunity: from C-reactive protein to the long pentraxin PTX3. J Clin Immunolo 28(1): 1-13.
- Misra HP, Fridovich I (1972) The Role of Superoxide Anion in the Autooxidation of Epinephrine and Simple Assay for Superoxide Dismutase. Journal of Biology and Chemistry 247(54): 3170-3175.
- Narmada F, Shalini W, Roshan N, Chaturaka R (2016) Protein carbonyl as a biomarker of oxidative stress in severe leptospirosis and its usefulness in differentiating leptospirosis from Dengue infections. PLos ONE 11(6): e0156085.
- Nuttall FQ (2015) Body Mass Index Obesity, BMI, and Health A Critical Review. Nutrition Today 50 (3): 117-128.
- Ogden CL, Fryar CD, Carroll MD, Flegal KM (2004) Mean body weight, height, and body mass index, United States 1960–2002. Adv Data 347: 1-17.
- Pacher P, Beckman J, Liaudet L (2007) Nitric oxide and peroxynitrite in health and dieases. Physiol. Rev 87(1): 315-424.
- Parthasarath S, Raghavamenon A, Garelnabi O, Santanam N (2012) Oxidized low density lipoprotein. Method Mol Biol 601: 403-417.
- Pfeiffer NE, McGuire TC, Bendel RB, Weikel JM (1977) Quantification of bovine Immunoglobulins: Comparison of Single Radial Immunodiffusion, Zinc Sulfate Turbidity, Serum Electrophoresis and Refractometer Methods. American Journal of Vetenary Research 38 (5): 693-698.
- Prokic MD, Paunovic MG, Matic MM, Djordjevic NZ, Ognjanovic BI, et al. (2014) Prooxidative Effects of Aspartame on Antioxidant Defense Status in Erythrocytes of Rats. Journal of Bioscience 39(5): 859-866.
- Qiwei W, Jun R, Stephanie M (2015) Monocyte Chemotactic protein-1Regulates Macrophage Cytotoxicity in Abdominal Aortic Aneurysm. Journal Prone 13(71): 53-56.
- Ridker PM (2003) Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation 10(7): 363-369.
- Romero-Corral A, Somers VK, Sierra-Johnson J (2010) Normal weight obesity: a risk factor for cardiometabolic dysregulation and cardiovascular mortality. Eur Heart J 31(6): 737-746.
- Sabine D, Massimo CF, Clemens N, Alexei N, Peter RG, et al. (2007) Cutting Edge: Trans-Signaling via the Soluble IL-6R Abrogates the Induction of FoxP3 in Naive CD4 and CD25 T Cells. The Journal of Immunology 179(4): 2041-2045.
- Sapna P, Shah UH, Mathur AH, Sharma S (2010) Department of Pharmacology, Army College of Medical Sciences. Journal of Pharmacology & Pharmacotherapeutics 2(21): 123-135.
- Tunnicliffe JM, Shearer J (2008) Coffee, Glucose Homeostasis, and Insulin Resistance: Physiological Mechanisms and Mediators. Applied Physiology, Nutrition and Metabolism 33(6): 1290-1300.
- Wu JN, Ho SC, Zhou C, Ling WH, Chen WQ (2009) Coffee Consumption and Risk of Coronary Heart Diseases: A Meta-Analysis of 21 Prospective Cohort Studies. International Journal of Cardiology 137(3): 216-225.

 Research Article
Research Article