Abstract
The infection with the new Coronavirus generated important socio-economic transformations, with profound economic implications, but also a lot of concern, due to evolutionary clinical complications and lack of specific treatment. Given that majority of cases of infection remain asymptomatic or evolve with mild symptoms, countries are seeking to assess the spread of the infection in the population through seroprevalence studies. This study aimed to estimate the degree of spread of SARS-CoV-2 infection in the Romanian population. The population enrolled in this study was selected from people presenting conjuncturally at selected laboratories. Chemiluminescence (CLIA) technology was used to detect SARS-CoV-2-specific IgG antibodies in blood samples. From June to October 2020, 19597 samples were collected. out of which, 6.19% (95%CI 5.85:6.53) were positive for SARS-CoV-2. The results suggest that a significant proportion of cases remains undiagnosed and unreported, considering that the notification rate at end October was 1.27%.
Keywords: Seroprevalence; COVID-19; Romania; Population; IgG Antibodies; Chemiluminescence
Abbreviations: NE: North-East; SE: South-East; S: South, SW: South-West, W: West, NW: North-West, C: Center, BI: Bucharest- Ilfov
Introduction
The infection with the new Coronavirus generated important socio-economic transformations, through social distancing measures, with profound economic implications, but also a lot of concern, due to evolutionary and clinical complications and lack of specific treatment. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) associated disease – 2019 (COVID-19) has spread globally, affecting in one year and half over 170 million people from more than 180 countries or regions, leading to a global pandemic with a fatality rate of 2.1% [1]. The laboratory diagnosis of suspected COVID-19 clinical / contact cases is based on the detection of SARS-CoV-2 viral genome by qRT-PCR assays. However, asymptomatic or mild COVID-19 infections remain undiagnosed, therefore the burden (incidence and spread) of SARS-CoV-2 infection can be underestimated, affecting the implementation and efficiency of infection control and prevention measures. Given this limitation, countries are seeking to assess the spread of the infection in the population through prevalence studies conducted on study groups which are representative for the general population [2,3].
The surveys conducted in the first half of the year 2020 in different countries or geographical regions on populations of different sizes revealed different seroprevalence rates, ranging from <0.1% to more than 20% and that it can increase over time during longitudinal follow-up. In Europe, the seroprevalence reported by different countries was in decreasing order Italy (11.0%) [4], Switzerland (weekly seroprevalence rate of 4.8% to 10.8% during five weeks) [5], France (between 3.8 and 10% in different regions) (2), Spain (4.6%) [6], Denmark (1.9%) [7], Greece (0.42%) [8]. In USA, a great variation of seroprevalence was reported for different geographical regions (1.0% - 31.5%) [9,10], while for Brazil the rate was 3.8% [11]. In South America, Chile reported a seroprevalence of 13,4 - 16% [12]. In Africa, Kenya reported a crude seroprevalence of 5,6% and a study done in Alzintan City of Libya presented a seroprevalence of 2,74% [13,14].
In Asia, the highest rates were reported for Pakistan (15.6- 37.7%) [15], Guilan province, Iran (22%) [16], in China different serological studies reported positivity rates ranging from 0.6% in Chengdu, Sichuan to 3.8% in Wuhan, Hubei [17], while the lowest rates were recorded in Malaysia (0.4 - 0.6%) [18] and South Korea (0.07%) [19]. Japan reported 3.3% seroprevalence in Kobe [20] and a cumulative case detection ratios (2.6 – 8.7%) at 3 prefecture-level seroprevalence (Tokyo, Osaka and Miyagi) [21]. All studies reported a higher seroprevalence rate in males, although the differences are not statistically significant [22]. Considering the large variation of seroprevalence among different populations, filling the gap with data from different geographical regions is needed in order to better evaluate the burden of COVID-19 pandemic. This study reports for the first time the results of a seroprevalence survey performed in the Romanian population, to estimate the degree of spread of SARSCoV- 2 infection and to substantiate the measures to respond to the COVID pandemic that will be adopted at the level of the Romanian health care system for the next period.
Material and Methods
In this study, people that presented themselves conjuncturally at selected laboratories have been invited to participate in the seroprevalence survey. The participating laboratories were selected from each of the 42 counties of Romania.
Study Design and Participants
A cross-sectional study was performed to assess the SARSCoV- 2 antibody seropositivity prevalence. The study used a nonprobability sampling method, known as convenience sampling. The sampling strategy had two steps: the selection of laboratories and the selection of persons. The inclusion criteria for the laboratories were the following: either public or private facilities, with high addressability (over 40000 samples per year) and serving ambulatory patients (non-hospitalized). Based on these criteria, each of the 42 County Public Health Directorates over the country selected between 3 and 5 laboratories to participate in the study (except Bucharest Public Health Directorate, which selected 9 laboratories). Inclusion and exclusion criteria for the enrolment of the study subjects were also defined. In order to be selected, people from all ages that presented themselves conjuncturally at the selected laboratories for check-ups were invited to participate in the study. They should not present signs of symptoms of respiratory infection or requested to be tested for Covid-19. The participants to the study were selected based on a sampling step, and only individuals who expressed their informed consent to participate in the study were enrolled.
If a person qualified in the sampling step did not agree to participate in the study, the next person was asked if willing to be enrolled. The data collection took place between July and October 2020. The participants had to sign an informed consent to be included in the study (for children the consent was signed by the parent/legal representative). The participants had also to provide their demographic information, that included age, gender, city of residence and personal pathologic history. The seroprevalence analysis involved residual serum obtained from these individuals. The size of the study sample was calculated using the EpiInfo 7 program, for obtaining regional and decadal age-group representation. The regional sample for a specific agegroup was proportionally allocated for the counties in the region, considering their total population for the corresponding age-group. The resident population of Romania from July 1, 2018, on decadal age groups was used, with an expected frequency of SARS-CoV-2 infection in Romania of 50% on each age group, an accuracy of 95% CI, error accepted 5 % and 5% losses accepted for each age group in the region.
Procedures
All the serum samples of the enrolled participants were analyzed by the National Institute of Public Health laboratory, using a chemiluminescent technology (CLIA) based assay to detect the anti-SARS CoV-2 antibodies of the IgG type. The samples were kept at temperatures between minus 12 and minus 20 degrees Celsius. Transportation of residual serum samples was done using refrigeration machines and, exceptionally, isothermal bags with ice packs. The quality criteria for the serum samples were the following: blood samples collected in biochemistry vacuums, without anticoagulant, with or without separating gel; samples with a serum volume of 0.5-1 ml for the age group 0-14 years and 1-2 ml in people over 14 years. The residual serum from people that were suspected of Covid-19 and those presenting jaundice, haemolysis or superinfection (with flakes or veil) were not considered.
Ethics Statement
The existing study protocol was reviewed and accepted by the Scientific Council of the National Institute of Public Health - Research Ethics Committee. The seroprevalence study was performed in full compliance with the principles of ethics and confidentiality of personal data. Written informed consent was obtained from all eligible for enrolment individuals, while all professionals involved in the collection, retrieval and storing of data have signed a confidentiality agreement.
Results
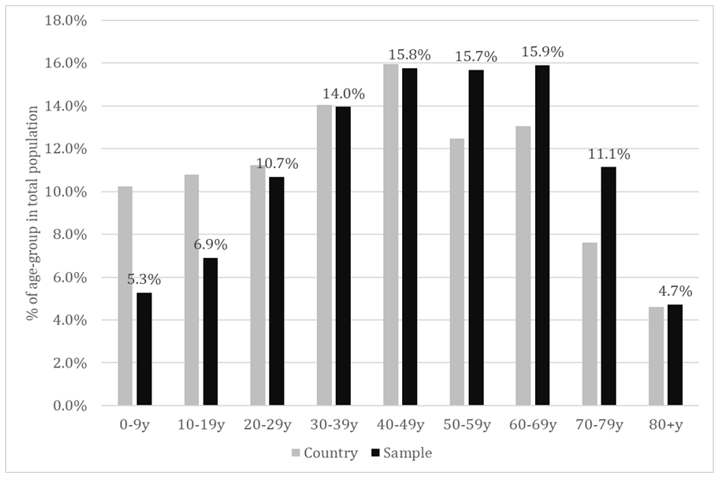
Of all the individuals that presented themselves at the selected laboratories across 8 regions of the country, 19738 agreed to participate in this study and 19597 provided a serum sample for which a CLIA result of anti-SARS-CoV-2 IgG specific antibodies was available. Males represented 36.2% of the total study population and this could be probably associated to the higher health-related concern of females in general, considering that the selection was conjunctural (people addressing themselves for different blood tests). The sample population had a mean age of 46.61±21.08 years and a median age of 48 years. The proportion of each decadal age-group is shown in Figure 1. As could be noticed, the young age-groups were seriously under-represented, meanwhile the agegroups 50-59y, 60-69y and 70-79 y were slightly over-represented (last-one in particular).
Figure 1: Proportion of the decadal age-groups in total population – sample versus country population.
Seroprevalence at National Level
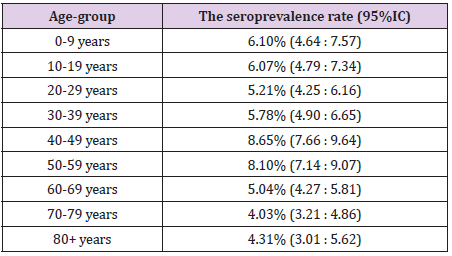
Overall, we found 1213 positive IgG samples in the study population, resulting in a seroprevalence rate of 6.19% (95%CI: 5.85:6.53). The seroprevalence rate by age-groups at national level is shown in Table 1. The level of protection was similar in children and young adults (slightly higher in children, but statistical significance was not met). The middle aged adults, especially the age-group 40-49 years showed a significantly higher level of protection. Population aged 60+ years seemed to be less protected compared to both adults and children. A statistically lower level of seroprevalence was revealed between each elderly age-group compared to middle-age adult population. A slight difference in seroprevalence was found compared to children and young adults, but this did not meet the statistical significance. We found also differences within the elderly groups. The seroprevalence seemed to be lower over the age of 70 years, compared to age-group 60 – 69, but, again, this difference did not meet the statistical significance.
Seroprevalence by Regions
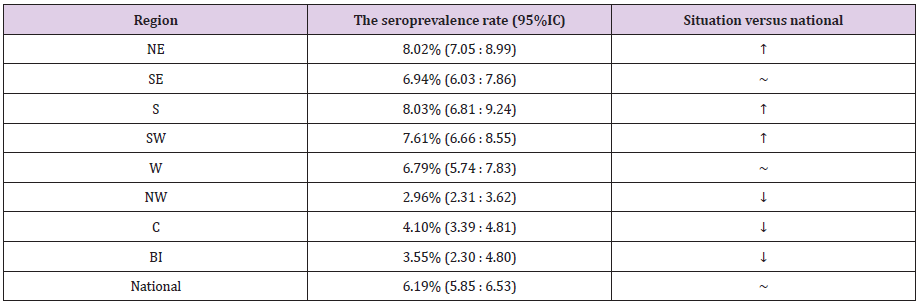
Romania is divided in eight region: North-East (NE), South- East (SE), South (S), South-West (SW), West (W), North-West (NW), Center (C) and Bucharest-Ilfov (BI) – the last-one including the capital city of Bucharest. By comparing the regions with the national rate, we found significantly higher prevalence in NE, S and SW, and significantly lower one in NW, C and BI (Table 2).
Seroprevalence by Age-Groups – Regional Versus National Level
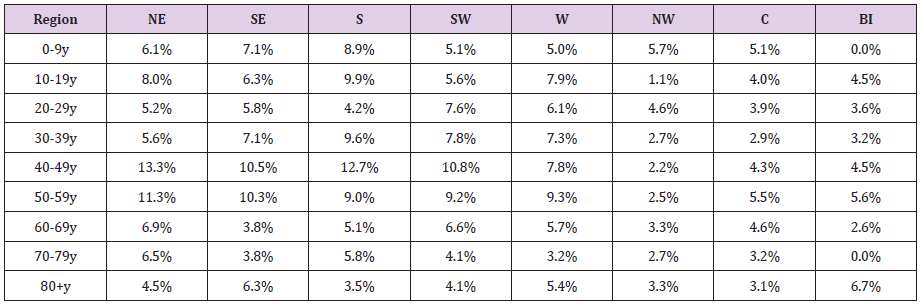
The seroprevalence by age-groups in the regions is shown in Table 3. Although the seroprevalence for each age group registered some variations among regions, significant differences compared to the national level were found only in limited cases. Thus, we found significantly lower seroprevalence rates compared to the national level in the regions NW (age-groups 10-19y and 30-59y) and Centre (age groups 30 – 39y and 40-49y). The only situation with a significantly higher level of protection was age-group 40-49y, in the NE region.
Seroprevalence in the Capital Region (BI)
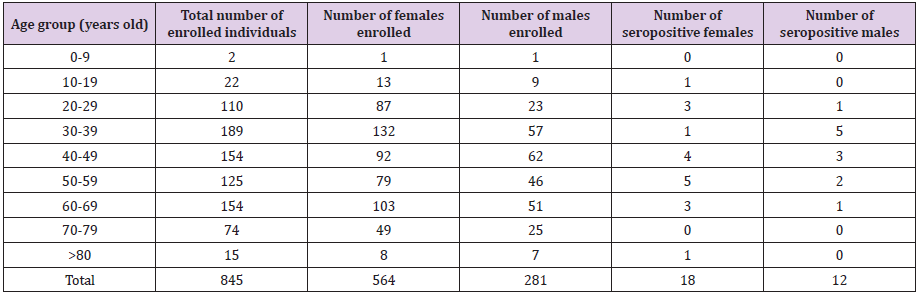
The enrolment rate in the Bucharest-Ilfov region was from far very poor (23% of planned). Table 4 provides details about the number and age of participants in Bucharest. Out of 845 participants, 30 tested positive for SARS-CoV-2-specific IgG antibodies, meaning a seroprevalence of 3.55% (2.30:4.80). A very limited number of cases was enrolled in the extreme age-groups (children and elderly population) and nonpositive case has been identified in age-groups 0-9y and 70-79y. The proportion of males was 33.3%, slightly lower compared to the national proportion (36.2%), but without statistical significance (p=0.081, Chi Square test). From the total positive cases, 18 were females and 12 were males. The enloled and positive cases are shown in Table 4.
Discussion
Given that the vast majority of infection cases remains asymptomatic, countries are seeking to assess the spread of the infection in the population through seroprevalence studies with representation for the general public. The aim of this study was to estimate the degree of spread of SARS-CoV-2 infection in the Romanian population. In this purpose, we have assessed, using a chemiluminescence immunoassay, the anti-SARS-CoV-2 IgG antibodies, as they last longer than IgM and therefore, play a crucial role in assessing the real prevalence of the virus [23]. SARS-CoV-2 invades human cells by binding the spike protein to the membrane protein receptor of the cell. The genome of this virus encodes four key proteins - spike (S), nucleocapsid (N), envelope (E) and membrane (M) [24-27]. As the spike protein is involved in the first step of the infectious process, represented by the interaction with specific receptors, followed by virus internalization in the infected cells, there are many assays that detect the specific antibodies anti-S protein of SARS-CoV-2. Chemiluminescence immunoassay represents an indirect detection method of the anti-SARS-CoV-2 antibodies [28].
It can detect either IgM or IgG in serum [29]. Different countries tested the performance of CLIA, all indicating good specificity, sensitivity and its convenience for sampling [29-31]. Other studies used this method on a specific population to report the seroprevalence: private healthcare group in Fukushima Prefecture, Japan [32]; elite football players in Germany [33]; multicenter, primary care, and emergency care facilities in North Carolina [34]. The findings in this seroprevalence study for SARS-CoV-2 suggest that the prevalence of IgG antibodies against the Spike protein of SARS-CoV-2 is over 6% in Romania. However, according to the official data reported from the surveillance system, the cumulated notification rate for confirmed COVID-19 cases reached to 1.27% at end October 2020, when our study was finished. Our results support the data published regarding the lower proportion of COVID cases which are generally requiring health care, based on the severity of their symptoms The overall seroprevalence in Romania was lower than that recorded in Sweden, but higher than reported in Germany and Spain [2]. However, it should be noted that the specified studies presented a number of differences, regarding the number of participants, time frame and the methods that were used to evaluate the presence of antibodies.
The more modest seroprevalence rate among elderly could be a reason for consideration in the next planning phase for controlling the pandemic. Also we found interesting and significant geographical variations among regions, which could be an argument in favour of adopting public health interventions tailored to the epidemiological situation in the region, even with particularization for the smallest territorial units. Our study has a number of limitations. Although convenience sample is a common strategy used by many researchers, it can provide biased results because this method has the possibility to over/underrepresent a population [35]. The response rate to the study invite achieved lower levels in extreme age-groups. This is normal, because generally the parents could be reluctant or hesitant in agree the enrolment of their children in surveys. On the other hand, the children are less likely to perform blood tests compared to the adults, thus their enrolment was more difficult. As for the elderly, due to the epidemiological situation, they might avoid or postpone their usual blood tests. Women were represented in a higher proportion than men in this study, meaning that women could be more interested in participating in surveys, or more active in general, in investigating their health status.
Conclusion
Our study suggests that the real number of individuals infected with SARS-CoV-2 in Romania exceeds by around five times the number of reported cases confirmed by PCR. Therefore, data on seroprevalence are very important for understanding the magnitude and distribution of the pandemic at country level. Repeating the study after the vaccination campaign could provide strong indications about the further needs of public health interventions.
Institutional Review Board Statement
The study protocol was approved by the Scientific Council of the National Institute of Public Health - Research Ethics Committee.
Informed Consent Statement
Informed consent was obtained from all individuals who voluntarily decided to participate in this study.
Acknowledgement
The authors would like to express their gratitude for the efforts of the staff from laboratories and district public health directorates to collect and analyse the samples. Also we express our high appreciations to all the persons that agreed to be enrolled in the study.
References
- (2021) WHO. Coronavirus Disease (COVID-19) Situation Reports.
- Eckerle I, Meyer B (2020) SARS-CoV-2 seroprevalence in COVID-19 hotspots. Lancet 396(10250): 514-5.
- Ko JH, Joo EJ, Kim SH, Kim YJ, Huh K, et al. (2020) Clinical application of rapid diagnostic test kit for SARS-CoV-2 antibodies into the field of patient care. J Microbiol Immunol Infect 54(1): 97-100.
- Vena A, Berruti M, Adessi A, Blumetti P, Brignole M, et al. (2020) Prevalence of Antibodies to SARS-CoV-2 in Italian Adults and Associated Risk Factors. J Clin Med 9(9): 2780.
- Stringhini S, Wisniak A, Piumatti G, Azman AS, Lauer SA, et al. (2020) Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet 396(10247): 313-319.
- Pollán M, Pérez Gómez B, Pastor Barriuso R, Oteo J, Hernán MA, et al. (2020) Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 396(10250): 535-544.
- Erikstrup C, Hother CE, Pedersen OBV, Mølbak K, Skov RL, et al. (2021) Estimation of SARS-CoV-2 Infection Fatality Rate by Real-time Antibody Screening of Blood Donors. Clin Infect Dis 72(2): 249-253.
- Bogogiannidou Z, Vontas A, Dadouli K, Kyritsi MA, Soteriades S, et al. (2020) Repeated leftover serosurvey of SARS-CoV-2 IgG antibodies, Greece, March and April 2020. Eurosurveillance 25(31): 2001369.
- Havers FP, Reed C, Lim T, Montgomery JM, Klena JD, et al. (2020) Seroprevalence of Antibodies to SARS-CoV-2 in 10 Sites in the United States, March 23-May 12, 2020. JAMA Intern Med 180(12): 1776-1786.
- Naranbhai V, Chang CC, Beltran WFG, Miller TE, Astudillo MG, et al. (2020) High Seroprevalence of Anti-SARS-CoV-2 Antibodies in Chelsea, Massachusetts. J Infect Dis 222(12): 1955-1959.
- Filho LA, Szwarcwald CL, Mateos S de OG, de Leon ACMP, De Andrade Medronho R, et al. (2020) Seroprevalence of anti-SARS-CoV-2 among blood donors in Rio de Janeiro, Brazil. Rev Saude Publica 54: 69.
- Brault A (2021) Seroprevalence of SARS-CoV-2 in Chile.
- Uyoga S, Adetifa IMO, Karanja HK, Nyagwange J, Tuju J, et al. (2021) Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Kenyan blood donors. Science 371(6524): 79-82.
- Kammon AM, El Arabi AA, Erhouma EA, Mehemed TM, Mohamed OA (2020) Seroprevalence of antibodies against SARS-CoV-2 among public community and health-care workers in Alzintan City of Libya.
- Younas A, Waheed S, Khawaja S, Imam M, Borhany M, et al. (2020) Seroprevalence of SARS-CoV-2 antibodies among healthy blood donors in Karachi, Pakistan. Transfus Apher Sci 59(6): 102923.
- Shakiba M, Nazari SSH, Mehrabian F, Rezvani SM, Ghasempour Z, et al. (2020) Seroprevalence of COVID-19 virus infection in Guilan province, Iran. medRxiv. medRxiv.
- Xu X, Sun J, Nie S, Li H, Kong Y, et al. (2020) Seroprevalence of immunoglobulin M and G antibodies against SARS-CoV-2 in China. Nat Med 26(9): 1494.
- Sam I, Chong YM, Tan CW, Chan YF (2021) Low postpandemic wave SARS‐CoV‐2 seroprevalence in Kuala Lumpur and Selangor, Malaysia. J Med Virol 93(2): 647-648.
- Noh JY, Seo Y Bin, Yoon JG, Seong H, Hyun H, et al. (2020) Seroprevalence of anti-SARS-CoV-2 antibodies among outpatients in Southwestern Seoul, Korea. J Korean Med Sci 35(33): e311.
- Doi A, Iwata K, Kuroda H, Hasuike T, Nasu S, et al. (2020) Seroprevalence of novel coronavirus disease (COVID-19) in Kobe, Japan. medRxiv. medRxiv.
- Yoshiyama T, Saito Y, Masuda K, Nakanishi Y, Kido Y, et al. (2021) Prevalence of SARS-CoV-2-specific antibodies, Japan, June 2020. Vol. 27, Emerging Infectious Diseases. Centers for Disease Control and Prevention (CDC), pp. 628-631.
- Lai CC, Wang JH, Hsueh PR (2020) Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. International Journal of Infectious Diseases. Elsevier 101: 314-322.
- Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, et al. (2020) The role of antibody testing for sars-cov-2: Is there one? J Clin Microbiol 58(8): 1-7.
- Chen Q, He Z, Mao F, Pei H, Cao H (2020) Diagnostic technologies for COVID-19: a review. RSC Adv 10(58): 35257-35264.
- Jiang S, Hillyer C, Du L (2020) Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends in Immunology. Elsevier Ltd 41(5): 355-359.
- Rostami A, Sepidarkish M, Leeflang MMG, Riahi SM, Nourollahpour Shiadeh M, et al. (2020) SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clinical Microbiology and Infection Elsevier 27(3): 331-340.
- Mihaescu G, Carmen Chifiriuc M, Iliescu C, Ovidiu Vrancianu C, Ditu LM, et al. Microorganisms SARS-CoV-2: From Structure to Pathology, Host Immune Response and Therapeutic Management Microorganisms 8(10): 1468.
- Martinez Liu C, Martínez Acuña N, Arellanos Soto D, Galan Huerta K, Lozano Sepulveda S, et al. (2021) SARS-CoV-2 in Mexico: Beyond Detection Methods, Scope and Limitations. Diagnostics 11(1): 124.
- Hu F, Shang X, Chen M, Zhang C (2020) Joint Detection of Serum IgM/IgG Antibody Is an Important Key to Clinical Diagnosis of SARS-CoV-2 Infection. Can J Infect Dis Med Microbiol 2020: 1-5.
- Rodriguez Villalobos H, Kabamba Mukadi B (2021) Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID-19 patients. J Med Virol 93(3): 1465-1477.
- Lijia S, Lihong S, Huabin W, Xiaoping X, Xiaodong L, et al. (2020) Serological chemiluminescence immunoassay for the diagnosis of SARS-CoV-2 infection. J Clin Lab Anal 34(10): e23466.
- Kobashi Y, Shimazu Y, Nishikawa Y, Kawamura T, Kodama T, et al. (2021) The difference between IgM and IgG antibody prevalence in different serological assays for COVID-19; lessons from the examination of healthcare workers. Int Immunopharmacol 92: 107360.
- Mack D, Gärtner BC, Rössler A, Kimpel J, Donde K, et al. (2020) Prevalence of SARS-CoV-2 IgG antibodies in a large prospective cohort study of elite football players in Germany (May–June 2020): implications for a testing protocol in asymptomatic individuals and estimation of the rate of undetected cases. Clin Microbiol Infect 27(3): 473.
- Barzin A, Schmitz JL, Rosin S, Sirpal R, Almond M, et al. (2020) SARS-CoV-2 seroprevalence among a southern U.S. population indicates limited asymptomatic spread under physical distancing measures. MBio 11(5): 1-8.
- Tang et al (2008) 基因的改变NIH Public Access. Bone 23(1): 1-7.

 Research Article
Research Article