Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virological agent responsible for the current pandemic. SARS-CoV-2 has accounted for more than 232 million infections and over 4 million deaths worldwide. The production of several vaccines has significantly impacted the morbidity and mortality rates; however, until 70% percent of the global population is vaccinated, the virus will continue to spread. A member of the betacoronaviruses, it is widely known that SARS-CoV-2 is transmitted from person to person mainly through contaminated respiratory products and contaminated surfaces. Viral transmission mitigation strategies are sufficient to control the incidence of disease. Compared to other RNA viruses (e.g., influenza) SARS-CoV-2 contains a large genome of approximately 30 kb. Spike-ACE2 binding leads to viral fusion and penetration. Once the RNA genome is located in the host’s cell cytoplasm, the virus takes advantage of the host’s transcriptional and translational machinery. Translation of the viral genome produces accessory proteins, nonstructural proteins, and structural proteins. The spike protein, membrane protein, envelope protein, and nucleoprotein are the main structural proteins. Both accessory and nonstructural proteins serve anti-immunity functions. Additional research regarding viral entry will facilitate the development of therapeutics to treat COVID-19. The review article also discusses potential future research studies to elucidate critical information on SARSCoV- 2 cell entry mechanisms.
Keywords: COVID-19; SARS-CoV-2; Epidemiology; Replication
Abbreviations: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19: Coronavirus Disease 2019; SARS: Severe Acute Respiratory Syndrome; MERS: Middle East Respiratory Syndrome; CoV: Coronaviruses; ORFs: Open Reading Frames; NSPS: Nonstructural Proteins; TRS: Transcription Regulatory Sequences; RDRP: RNA-Dependent RNA Polymerase
SARS-CoV-2
The Coronavirus Disease 2019 (COVID-19) is the single most massive global health threat in over 100 years. Previous coronavirus outbreaks, severe acute respiratory syndrome (SARS) coronavirus, and the Middle East respiratory syndrome (MERS) coronavirus did not result in a significant number of deaths worldwide. Unfortunately, since COVID-19 first appeared on the epidemiological landscape, it has led to immeasurable sickness, suffering, economic problems, and loss of life in the United States [1,2]. COVID-19 has dramatically changed the way society functions, from the way we watch sporting events, work, matriculate, and vacation. COVID-19 will likely impact societal life long after the eradication of the pandemic. Coronaviruses (CoV) are members of the family Coronaviridae and infect domestic animals, wild animals, and humans. When viewed under a transmission electron microscope, these spherical viruses are named for their crown-like or solar corona-like appearance and are roughly 140-160 nm in size. There are four known genera of coronaviruses: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus [3]. Both the alphacoronaviruses and betacoronaviruses cause human diseases; the gammacoronaviruses and deltacoronaviruses are primarily found in birds. Since their emergence in the 1960s, virologists have been aware of seven members of the family Coronaviridae that cause illnesses in humans (229E, NL63, OC43, HKU1, SARS-CoV-1, MERS-CoV, SARS-CoV-2). Phylogenetic analysis reveals that both the alphacoronaviruses and the betacoronaviruses originated in bats and jumped to humans via zoonotic transfer. Civet cats (Paguma larvata) and dromedary camels (Camelus dromedarius) are the intermediate hosts for the original SARS and MERS, respectively [4]. The reservoir host for the latest coronavirus is unknown; however, amino acid analysis revealed that the spike glycoprotein for SARS-CoV-2 bears a 97% homology to pangolin coronaviruses, suggesting that it may be an intermediate host of the pandemic virus [5]. SARS-CoV-2 is a member of the betacoronaviruses. SARS-CoV-2, similar to other members of the family Coronaviridae, is a single-stranded (positive polarity) RNA enveloped virus [6].
Structure of SARS-CoV-2
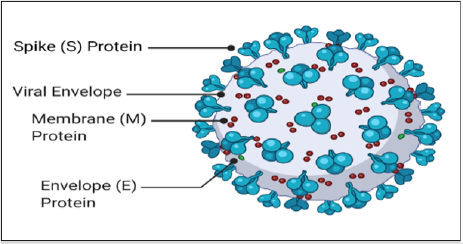
A genetic comparison of SARS-CoV-1 and SARS-CoV-2 revealed that their genomes share 80% homology. Figure 1 shows the critical structural features of SARS-CoV-2. The four structural proteins are the membrane protein (M), an envelope protein (E), nucleocapsid protein (N), and the spike glycoprotein (S) (Figure 1) [1]. The membrane protein is a transmembrane protein that connects the helical nucleocapsid to the nuclear envelope through its C-terminal region and represents the most abundant structural polypeptide. Envelope proteins maintain the lipid bilayer’s stability and play a role during viral maturation and virion release. Nucleocapsid proteins bind to and protect the viral genome. The nucleocapsid protein and RNA complex are found in the viral core. Spike glycoproteins are responsible for host cell attachment and viral cellular entry. Unbound S molecules are proproteins that convert the S glycoprotein into its active fusogenic state [7]. The SARSCoV- 2 viral lipid envelope, like other enveloped viruses, is derived from host cell membranes.
Figure 1: Spherical COVID-19 virus. The 100-200nm virus contains spike proteins, membrane proteins, and envelope proteins embedded in the viral membrane. The nucleoprotein and positive-sense single-stranded RNA is located inside the viral core beneath the viral membrane.
Epidemiology
Human-to-human transmission of the virus is accomplished via four main routes: respiratory droplet transmission, fomite transmission, fecal-oral transmission, and airborne aerosol microdroplet transmission [1]. Community transmission can occur via asymptomatic, pre-symptomatic, and post-symptomatic modalities for SARS-CoV-2, which is different from SARS-CoV-1 [8]. It is unclear if the domestic animal-to-human viral transmission is possible. Although evidence supporting aerosol microdroplet transmission is tenuous, the nature of the data supports the need for masks, adequate handwashing, social distancing, and proper dwelling ventilation as potential pandemic mitigation strategies. The basic reproductive number for COVID-19 is 2-4, which means that an infected person can infect 2-4 susceptible individuals. Highrisk individuals include the elderly and people with preexisting conditions, including high blood pressure, diabetes, obesity, and heart disease. Currently, rapid COVID-19 testing utilizes real-time polymerase chain reaction assays, in which healthcare professionals obtain blood, saliva, nasopharyngeal, fecal, oropharyngeal, sputum, and bronchoalveolar lavage samples and test the samples for SARSCoV- 2 genetic markers [6,8].
Genome
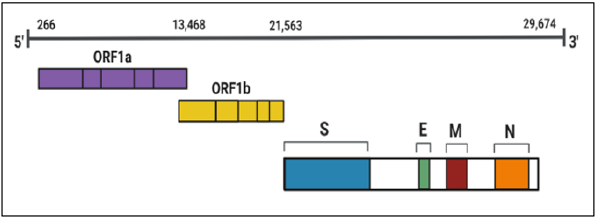
The SARS-CoV-2 genome (29.9 kb) consists of 14 open reading frames (ORFs) that encode 29 proteins (Figure 2). Accessory proteins, nonstructural proteins (nsps), and structural proteins comprise the proteome. ORF1a/ORF1b encodes the replicase complex. Proteolytic cleavage of the polyproteins produced by the translation of ORF1a/ORF1b gives rise to viral nonstructural proteins [6]. Nonstructural proteins function in genome replication, morphogenesis, and virulence. Accessory proteins are coronavirus specific and largely contribute to the pathogenesis of the novel coronavirus. Both the 5′ end and the 3′ end contain untranslated regions. A cap structure and a poly (A) tail are found on the 5′ and 3′ end, respectively [9]. The presence of transcription regulatory sequences (TRS) in the genome facilitates transcription of the viral genome and the production of subgenomic mRNAs, which leads to the generation of structural and accessory proteins.
Figure 2: SARS-CoV-2 genomic organization. The pandemic virus is composed of 30 kbp (kilobase pairs). The four major structural proteins: spike (S), envelope (E), membrane (M), and nucleocapsid (N) are mostly located at the 3’ end.
Replication
The ability of SARS-CoV-2 to effectively attach to host cells and subsequently enter cells is dependent on the binding of the virus’s spike glycoprotein to the complementary host cell surface receptor [10]. Viral cell entry also depends on the activation of fusogenic events that lead to membrane fusion of the virus and the host cell. It is believed that several cellular proteases such as TMPRSS2 (transmembrane serine protease 2) directly promote viral-host cell fusion, which allows the infiltration of the viral genome into the host cell. With the aid of the RNA-dependent RNA polymerase (RDRP), the pathogenic viral genome directs the synthesis of viral molecules (e.g., positive-strand RNA, structural proteins, accessory proteins, nonstructural proteins). Subsequent assembly of complete viral particles and expulsion completes the virus’s replication cycle, responsible for a staggering number of deaths (Figure 3). Investigating SARS-CoV-2 cell entry mechanisms at the molecular level may lead to discovering host-specific proteases, cellular factors, and protein trafficking molecules paramount for cellular infection.
Figure 3: Generalized SARS-CoV-2 replication strategy. Following attachment via the spike protein and ACE2 receptor, SARSCoV- 2 utilizes host cell metabolic machinery to produce progeny viral particles.
Cell Entry Research Studies
Investigating the fundamental virology of SARS-CoV-2, the invisible pathogenic culprit responsible for COVID-19, remains a high priority on the global scientific landscape. Future research projects that explore the underlying molecular mechanisms involved in viral entry into the intracellular host environment would greatly benefit the scientific community. Moreover, to gain a greater understanding of the specific molecular networks and signaling pathways associated with viral cell entry, microarray technology or next-generation RNA sequencing and bioinformatics analysis should be employed to analyze gene expression profiles during viral entry events in human cell types known to be susceptible to SARS-CoV-2 [11,12]. A detailed experimental examination of viral cell entry will enhance our comprehension of viral host range, viral replication, and immune evasion. It may also augment our knowledge of endocytosis and the cellular uptake of exogenous material across the Domain Eukarya. In the short term, an increased understanding of attachment, fusion, and penetration processes will help scientists to develop specific drugs to treat infections that work by disrupting essential replication mechanisms [13,14].
Conclusion
SARS-CoV-2 is the viral agent responsible for the deadly pandemic. The current viral pathogen is the second virus in the severe acute respiratory syndrome lineage to infect humans. While SARS-CoV-1 effectively killed humans in the early 2000s, SARS-CoV-2 has perfected the art of invading the human body and targeting multiple cell types. The enormous genome contained in the viral core presents unique challenges for the pathogenic virus. Because of its genome size, the virus engages in unusual molecular mechanisms. In general, viruses do not contain enzymes or other preformed molecules that enable the assembly of intact virus particles. However, SARS-CoV-2, like other RNA viruses, contains capacity to synthesize RNA-dependent RNA polymerase. The primary function of RDRP is to generate additional copies of RNA that are eventually packaged in the viral lipid bilayer. Future research should continue to focus on targeting the SARS-CoV-2 replication cycle. The aggressive study of the many biological processes employed by the virus will undoubtedly bring about the development of drugs to inhibit attachment, penetration, biosynthesis, maturation, and expulsion. The main strategy being employed regarding drug identification is therapeutic repurposing. Using this strategy, scientists can determine the efficacy of already approved drugs to stop the growth of the virus in human cells.
Acknowledgement
This work was supported by a grant funded by the National Science Foundation (HRD-1533536).
References
- Flowers L (2020) COVID-19 and the cytokine storm. Biomedical Journal of Scientific & Technical Research 29: 22644-22647.
- Kannan S, Shaik P, Sheeza A, Hemalatha K (2020) COVID-19 (Novel Coronavirus 2019) - Recent trends. European Review for Medical and Pharmacological Sciences 24(4): 2006-2011.
- Malik Y (2020) Properties of coronavirus and SARS-CoV-2. The Malaysian Journal of Pathology 42(1): 3-11.
- Wang L, Eaton B (2007) Bats, civets and the emergence of SARS. Current Topics in Microbiology and Immunology 315: 325-344.
- Zhang T, Wu Q, Zhang Z (2020) Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biology 30(7): 1346-1351.
- Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. Journal of Pharmaceutical Analysis 10(2): 102-108.
- Pillay T (2020) Gene of the month: The 2019-nCoV/SARS-CoV-2 novel coronavirus spike protein. Journal of Clinical Pathology 73(7): 366-369.
- Rothan H, Byrareddy S (2020) The epidemiology and pathogenesis of coronavirus disease (COVID19) outbreak. Journal of Autoimmunity 109: 1-4.
- Kim D, Lee J, Yang J, Kim J, Kim V, et al. (2020) The architecture of SARS-CoV-2 transcriptome. Cell 181(4): 914-921.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, et al. (2020) Cell entry mechanisms of SARS-CoV-2. Proceedings of the National Academy of Sciences of the United States of America 117(21): 11727-11734.
- Flowers L (2021) Understanding microbial infections using microarray technology. International Journal of Science and Research Archive 2: 131-135.
- Flowers L (2019) The use of PCR arrays to investigate human immune responses. Biomedical Journal of Scientific & Technical Research 21: 15847-15851.
- Gavriatopoulou M, Ntanasis-Stathopoulos I, Korompoki E, Fotiou D, Migkou M, et al. (2021) Emerging treatment strategies for COVID-19 infection. Clinical and Experimental Medicine 21(2): 167-179.
- Sreekanth Reddy O, Lai W (2021) Tackling COVID-19 using remdesivir and favipiravir as therapeutic options. Chembiochem 22(6): 939-948.

 Review Article
Review Article