Short Communication
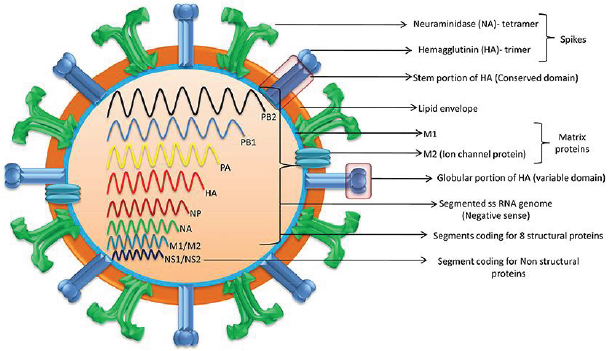
In the US, influenza (flu) causes 9 to 45 million illnesses, leading to 12,000 to 61,000 deaths annually (10.1001/jama.2020.14772). The World Health Organization estimates that annually there are about one billion infections, 3-5 million severe illnesses, and 300,000-500,000 deaths worldwide (10.1038/s41572-018- 0002-y). Influenza is caused primarily by influenza A and influenza B viruses [1]. Influenza A is the cause of pandemics. A schematic diagram of the influenza A virus is presented in Figure 1. Influenza will continue to be prevalent because current vaccines are safe but only 30-60% efficacious. In contradistinction, COVID-19 vaccines have an efficacy of about 90%. Additionally, unlike coronavirus, new zoonotic influenza strains intermittently migrate to humans. Influenza is a major part of “The New Normal.”
Co-infection
Overlapping spread of COVID-19 and influenza could be a major strain on the health care system [2]. Their coinfection is not common but may cause more severe disease. Influenza vaccination and therapy are important in addition to COVID-19 vaccination and therapy, especially in persons at increased risk.
Incubation
Influenza’s median incubation period is 2 days. Patients are infectious for a day before and 5 to 7 days after symptoms. COVID-19 has an incubation period of 4-12 days, a median of about 5 days [3]. Patients are most infectious from 2 days before symptom onset. Influenza symptoms peak in 3-7 days. COVID-19 symptoms peak in second or third week. The spread of both viruses is facilitated by transmission by asymptomatic patients.
Transmission
About one-half of influenza A cases are due to aerosol transmission (10.1038/s41598-019-38825-y). Adequate ventilation can reduce it. Used appropriately, surgical masks reduce the concentration of aerosolized influenza virus by about ten-fold and are adequate for prophylaxis against influenza (10.1016/j.jhin.2013.02.007; 10.7326/M20-3213). Transmission by respiratory droplets can also be reduced by masking [4-9]. Transmission by direct contact can be reduced by hand and general hygiene. COVID-19 is transmitted in a similar manner. Social distancing reduces transmission by all the mechanisms. According to the Centers for Disease Control and Prevention (CDC), the precautions utilized for COVID-19 reduced the positivity rate for influenza tests from 16.8% in 2019-20 to 0.15% in 2020-21. Influenza is less contagious and causes less severe disease than COVID-19. Quarantine recommended for COVID-19 patients is not necessary for those with influenza. As children play an important role in transmitting influenza, opening of schools is likely to increase transmission. Healthcare providers should take precautions to avoid infecting themselves and others.
Influenza-Like Illness (ILI)
CDC defines ILI as fever 100°F (38°C) or greater and cough or sore throat that is not due to another known cause such as streptococcal pharyngitis (strep throat). ILI’s causes can be benign such as common cold, i.e., nasopharyngitis caused by rhinovirus or other viruses. ILI’s severe causes include sepsis, meningitis, COVID-19, and SARS. Often there is an abrupt onset. SARS leads to severe disease in most of the infected persons. Influenza and COVID-19 cause mild disease in most of the infected persons (10.1016/S1473-3099(20)30484-9). SARS is currently not prevalent [10]. Severe disease due to COVID-19 and SARS occurs primarily in the elderly. Influenza is more evenly distributed across the age groups. ILI can cause immunosuppression, leading to bacterial pneumonia, necessitating antibiotic treatment (10.1097/ QCO.0000000000000347). After general anesthesia, children with influenza have a longer hospital length of stay and increased risk of requiring intensive care (10.1186/1471-2253-11-16). Routine surgery should be postponed for about four weeks in a patient with ILI. Unvaccinated patients should be offered influenza vaccination after the acute phase of ILI has passed and before the surgery. Before performing urgent surgery, severe causes of ILI should be excluded [11].
Upper Respiratory Tract Infection (URI) Excluding ILI
In cold weather URI is common, especially in children. Often it is due to a common cold, or noninfectious allergic or vasomotor rhinitis. Less commonly, it can be an early presentation of more serious illness including ILI, COVID-19, strep throat, and herpes simplex. Early in infection it is difficult to distinguish between different etiologies of URI. URI may cause sneezing, coughing, headache, malaise, rhinorrhea, sore throat, sinusitis, and bronchitis. Subsequently, bronchi may be hyperreactive for about six weeks. Pulmonary complications associated with surgery in a patient with URI are bronchospasm, laryngospasm, coughing, breath holding, postintubation croup, episodes of desaturation, atelectasis, and pneumonia. Anticholinergics and bronchodilators may not be beneficial. If ILI and other serious illnesses are unlikely, routine surgery can be performed with caution. Perioperatively, adequate hydration and humidification should be maintained. There are no pediatric or adult anesthesia closed claims that implicate URIs including influenza with serious adverse events.
Cardiac Effects
There is a very small incidence of viral myocarditis in patients with URI including ILI. It may lead to serious arrhythmias and refractory heart failure (10.1016/j.jcrc.2018.06.001; 10.1038/ s41569-020-00435-x). Cardiac abnormality should be excluded before performing non-emergent surgery.
Diagnostics Tests
These include nucleic acid amplification via polymerase chain reaction (PCR) and antigen-based immunological assays [12]. A PCR test can be performed even at the point-of-care with results available within an hour (10.1016/S2213-2600(20)30469-0). This can facilitate infection control and utilization of antiviral thrapeutics. It is especially useful for patients who have severe symptoms or are hospitalized. Test for COVID-19 may also be performed if indicated.
Influenza Vaccine
It is the best preventive measure. Despite moderate efficacy, it substantially reduces morbidity and mortality because of the high prevalence of influenza. It is recommended for anyone over 6 months of age. It is especially beneficial in the presence of age <2 years or >65 years, pregnancy, and pre-existing conditions (10.1001/jama.2020.14772). Lack of vaccination in pregnancy not only increases the risk to the mother but also increases risk of preterm birth, fetal death, infant respiratory infections, and hospital admission [13]. The T cell response vaccines elicit is substantially weaker than the antibody response. Children may need two doses of the vaccine, at least four weeks apart. The vaccine should be administered at least one week before surgery. It takes two weeks to develop full effect. As the protection wanes over time, mid- September to mid-October is preferred for vaccination. Influenza and COVID-19 vaccines may be administered together. The vaccine may be administered to surgical inpatients (10.7326/M15-1667).
Available Vaccines
The influenza virus mutates frequently. Quadrivalent vaccines protect against four of the currently most prevalent strains of influenza. The vaccines are altered every year for the predicted prevalent strains. Nine vaccines from four manufacturers are available in the US. Inactivated influenza vaccine is most commonly used. It is approved for persons above 6 months of age. As older individuals have a reduced response, vaccines that have a higher dose or are adjuvanted are recommended for persons above 65 years of age [14]. The vaccine is usually administered intramuscular, but a lower dose intradermal vaccine may be non-inferior (13-10.1001/ jamanetworkopen.2020.35693). Live-attenuated influenza vaccine is administered via nasal spray. It is approved for ages 2-49 years. It may be preferable in some situations such as vaccinating many persons in a community. It should be avoided if the patient or someone nearby has a suppressed immune system. Hence, it is not suitable for inpatients. Recombinant vaccine and cell culture vaccine do not contain egg products. They are especially suitable for persons who need to avoid eggs because of allergy or dietary preferences.
Future Vaccines
Universal vaccines that provide durable response against all influenza strains are in human trials (10.1038/s41591-020- 1118-7). These vaccines generate antibodies against the viral hemagglutinin protein stem (stalk) domain (HA2). Current vaccines generate antibodies against the immunodominant globular head domain (HA1), which is variable and mutates much more frequently [15]. Vaccines utilizing mRNA are also in human trials. They are likely to have greater efficacy but more side effects than current vaccines. A major advantage of the mRNA vaccines is that they can be readily modified to match mutations in the virus.
Benefits of Vaccination for Adults
Influenza vaccine reduces the risk of respiratory and cardiovascular adverse outcomes and mortality among adults, especially in the presence of pre-existing conditions and advanced age. This was confirmed in a meta-analysis of studies on all adults (10.1016/j.arr.2020.101124). Another meta-analysis found that vaccination reduced the risk of adverse cardiac outcomes, especially in patients with more severe cardiac disease (10.1001/ jama.2013.279206). Preoperative vaccination is beneficial. A large study of elderly patients who had major surgery found preoperative vaccination reduced by about one-half the risk of pneumonia, intensive care admission, and death (10.1093/infdis/jix616). Patients also had shorter hospital stays and reduced resource utilization.
Influenza Therapeutics
These include neuraminidase inhibitors oseltamivir, zanamivir, peramivir, and laninamivir; cap-dependent endonuclease inhibitor baloxavir; and matrix protein M2 ion channel blockers (10.1001/ jamanetworkopen.2021.19151). They attenuate viral replication. They provide postexposure prophylaxis. When started within 2 days of symptom onset, they reduce duration and severity of the disease, and complications. They also reduce transmission of influenza virus. However, their efficacy is limited, especially in patients with serious illness [16]. They are expensive and not widely utilized. Although influenza and COVID-19 have similar initial symptoms, their therapeutics are different. Thus, dexamethasone reduces mortality for hospitalized COVID-19 patients on respiratory support but may increase mortality for hospitalized influenza patients (10.1001/ jama.2020.15260).
Influenza Pandemics
They are usually caused by zoonotic influenza A virus strains migrating to humans. The 1918 “Spanish flu” pandemic caused by influenza A H1N1 virus led to more than 40 million deaths worldwide. H1 denotes haemagglutinin subtype 1 and N1 denotes neuraminidase subtype 1. The pandemics of 1957, 1968 and 2009 were caused by influenza A H2N2, H3N2, and H1N1 viruses, respectively [17,18]. The 2009 “swine flu” influenza A H1N1 virus that originated from pigs was antigenically different from previously dominant influenza A H1N1 viruses. It caused 150,000- 600,000 deaths worldwide. Avian influenza “bird flu” caused by H5N1 and H7N9 is not prevalent. Overlapping occurrence of influenza pandemic and COVID-19 could be devastating. Vigilance and prompt action are essential to prevent zoonotic influenza A virus strains from migrating to humans.
Conclusion
Influenza is a prevalent respiratory disease that will continue to affect anesthesia practice in the foreseeable future. Vaccination reduces risk. With appropriate management, the risk of adverse outcomes is low.
References
- Solomon DA (2020) Seasonal Influenza Vaccination. JAMA 324(12): 1362.
- Krammer F, Smith GJD, Fouchier RAM, Peiris M, Kedzierska K, et al. (2018) Influenza. Nat Rev Dis Primers 4(1): 3.
- Smieszek T, Lazzari G, Salathe M (2019) Assessing the Dynamics and Control of Droplet- and Aerosol-Transmitted Influenza Using an Indoor Positioning System. Sci Rep 9(1): 2185.
- Zhang XS, Duchaine C (2021) SARS-CoV-2 and Health Care Worker Protection in Low-Risk Settings: a Review of Modes of Transmission and a Novel Airborne Model Involving Inhalable Particles. Clin Microbiol Rev 34(1): e00184-20.
- Petersen E, Koopmans M, Go U, Davidson H Hamer, Nicola Petrosillo, et al. (2020) Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis 20(9): e238-e244.
- Martin-Loeches I, Van Someren Greve F, Schultz MJ (2017) Bacterial pneumonia as an influenza complication. Curr Opin Infect Dis 30(2): 201-207.
- Spaeder MC, Lockman JL, Greenberg RS (2011) Impact of perioperative RSV or influenza infection on length of stay and risk of unplanned ICU admission in children: a case-control study. BMC Anesthesiol 11: 16.
- Hekimian G, Jovanovic T, Brechot N (2018) When the heart gets the flu: Fulminant influenza B myocarditis: A case-series report and review of the literature. J Crit Care 47: 61-64.
- Tschope C, Ammirati E, Bozkurt B, Alida L P Caforio, Leslie T Cooper, et al. (2021) Myocarditis and inflammatory cardiomyopathy: current evidence and future directions. Nat Rev Cardiol 18(3): 169-193.
- Clark TW, Beard KR, Brendish NJ, Alida L P Caforio, Leslie T Cooper, et al. (2021) Clinical impact of a routine, molecular, point-of-care, test-and-treat strategy for influenza in adults admitted to hospital (FluPOC): a multicentre, open-label, randomised controlled trial. Lancet Respir Med 9(4): 419-429.
- Tartof SY, Qian L, Rieg GK, Lina S Sy, Hung Fu Tseng, et al. (2016) Safety of Seasonal Influenza Vaccination in Hospitalized Surgical Patients: A Cohort Study. Ann Intern Med 164(9): 593-599.
- Egunsola O, Clement F, Taplin J, Liza Mastikhina, Joyce W Li, et al. (2021) Immunogenicity and Safety of Reduced-Dose Intradermal vs Intramuscular Influenza Vaccines: A Systematic Review and Meta-analysis. JAMA Netw Open 4(2): e2035693.
- Nachbagauer R, Feser J, Naficy A, David I Bernstein, Jeffrey Guptill, et al. (2021) A chimeric hemagglutinin-based universal influenza virus vaccine approach induces broad and long-lasting immunity in a randomized, placebo-controlled phase I trial. Nat Med 279(1): 106-114.
- Cheng Y, Cao X, Cao Z, Chenjie Xu, Li Sun, et al. (2020) Effects of influenza vaccination on the risk of cardiovascular and respiratory diseases and all-cause mortality. Ageing Res Rev 62: 101124.
- Udell JA, Zawi R, Bhatt DL, Maryam Keshtkar-Jahromi, Fiona Gaughran, et al. (2013) Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA 310(16): 1711-1720.
- Liu WC, Lin CS, Yeh CC, Hsin-Yun Wu, Yuarn-Jang Lee, et al. (2018) Effect of Influenza Vaccination Against Postoperative Pneumonia and Mortality for Geriatric Patients Receiving Major Surgery: A Nationwide Matched Study. J Infect Dis 217(5): 816-826.
- Liu JW, Lin SH, Wang LC, Lin-Chien Wang, Jen-Ai Lee, et al. (2021) Comparison of Antiviral Agents for Seasonal Influenza Outcomes in Healthy Adults and Children: A Systematic Review and Network Meta-analysis. JAMA Netw Open 4(8): e2119151.
- Rubin R (2020) What Happens When COVID-19 Collides With Flu Season? JAMA 324(10): 923-925.

 Short Communication
Short Communication