Abstract
Background: The FDA released guidelines in 2014 addressing multiple reported
neurological complications associated with epidural steroid injections. FDA findings
were generalized and did not specify type of steroid. Various cohort studies and
systematic reviews have compared particulate versus non-particulate corticosteroids,
showing minimal differences in outcomes. Case series and systematic reviews revealed
multiple cases of neurological compromise with particulate steroid injections, but
none with non-particulate steroid injections. Despite these two premises, practitioners
have strongly skewed towards utilizing particulate steroids for transforaminal epidural
steroid injections. The Spine Intervention Society (SIS) published guidelines in 2019
taking a stronger stance in favor of the use of non-particulate steroids. This review
aims to explore the literature on this controversial subject.
Objectives: This study aims to review the current literature on efficacy for
particulate versus non-particulate corticosteroid transforaminal epidural steroid
injections in relation to the potential for neurological complications.
Design: Narrative review
Methods: Literature review of all available evidence was conducted via Google
Scholar and PubMed databases. Search terms included: transforaminal epidural steroid
injection complications and effectiveness of particulate versus non-particulates in
transforaminal epidural steroid injections. Studies of exclusively interlaminar epidural
steroid injections were excluded.
Results: There are various studies and case reports citing neurological
complications associated with particulate transforaminal steroid injections, ranging
from spinal infarct and blindness to death. This is contrasted with no reported cases for
dexamethasone. Multiple cohort studies and systematic reviews have been completed
comparing the efficacy of particulate steroids to non-particulates. There appears to be
a minimal but greater short-term benefit of particulates over non-particulates. This
benefit disappears after 2 months in studies that include long-term follow up.
Limitations: This is a limited narrative literature review comparing data ranging
from case reports to randomized controlled trials. There was no standardization or
secondary statistical data analysis. Further meta-analyses could focus on pooling data
to draw broader conclusions.
Conclusions: Numerous studies have shown minimal greater short-term benefit
of particulates over non-particulates; however, after factoring in possible neurological
risk with particulates, scales have heavily tipped in favor of use of non-particulate
steroids. These findings support the use of non-particulate steroids in the context of
documented safety concerns with particulate steroids.
Keywords: Spine Interventional Society; Transforaminal Epidural Steroid Injection; Non-Particulate Corticosteroids; Particulate Corticosteroids; Neurological Complications; Dexamethasone; Triamcinolone; Methylprednisolone; Betamethasone
Abbreviations: TFESI: Transforaminal Epidural Steroid Injections; NPS: Non- Particulate Steroid; PS: Particulate Steroids; FAERS: FDA’s Adverse Event Reporting System; SIS: Spine Intervention Society
Introduction
Transforaminal epidural steroid injections (TFESI) are widely
used interventional procedures for diagnosis and treatment
of spinal nerve pain and pathologies. Two broad categories of
corticosteroids exist: those that contain particles and those
without. The most well-known non-particulate steroid (NPS) is
dexamethasone and the most popular particulate steroids (PS)
are betamethasone, triamcinolone and methylprednisolone [1].
Steroid categorization is based on solubility properties in water as
well as the propensity to aggregate in various solutes. PS range in
size from 0.5-100um, though aggregates can reach sizes of 1000um
[2]. In contrast, the average red blood cell is 7um [3]. Interestingly,
betamethasone can fall into both categories based on preparation.
The acetate version is considered a PS whereas the sodium
phosphate version is considered NPS [2]. The significance of these
particles and aggregates lies within the embolic risk if inadvertently
injected intravascularly, leading to spinal or brain ischemia. In
2014, the FDA investigated multiple case reports of catastrophic
post-injection complications including paralysis, spinal infarct,
ischemic stroke and death [4]. The FDA’s Adverse Event Reporting
System (FAERS) database and the medical literature between 1997
and 2014 were reviewed. A total of 90 serious complications after
epidural steroid injections were identified. Type of corticosteroids
used were generalized and never specifically identified by the
FDA. However, subsequent review of the literature revealed that
these complications occurred with particulate steroids. In 2014,
the FDA required relabeling of PS products to include reports of
serious medical events [4-6]. Despite the new FDA warnings, most
practitioners still utilize PS for TFESIs likely due to availability,
training, physician preference and a persistent belief that PS are
superior in efficacy. Proponents of PS point to the theoretical
benefit of a local depot effect where larger particles absorb slower
due to their local accumulative nature. These slower diffusion
rates would then result in longer lasting effects [7]. Proponents of
PS also suggest that intrinsic risks are associated with all spinal
injections including vasospasm, vertebral artery dissection and
air embolization. Vasospasm can occur after arterial irritation due
to alpha-1 adrenergic activation and resultant vasoconstriction.
If prolonged enough, this vasoconstriction can cause ischemia.
Dissection can occur with any needle trauma separating the tunica
intima from the media. This can then shunt blood into the false
lumen causing distal arterial ischemia.
Air embolization can occur with any interventional procedure
that involves injections. If the syringe is not correctly prepared,
air particles can be injected into the artery causing embolization
of the artery [8-10]. There are few reports in the literature of
these types of adverse events [11-13]. Though there has been a
longstanding divide between those who advocate for PS vs NPS
for transforaminal epidural steroid injections, the issue has come
to the forefront with the introduction of new Spine Intervention
Society (SIS) guidelines [14]. The purpose of this study is to review
current evidence regarding efficacy and potential complications of
PS versus NPS.
Methods
A literature review of available evidence was conducted utilizing Google Scholar and PubMed databases. Key search terms included: transforaminal epidural steroid injections, complications, efficacy, particulate steroids, non-particulate steroids, dexamethasone, methylprednisolone, betamethasone and triamcinolone. Studies that involved exclusively interlaminar epidural steroid injections were excluded. No limitations for publication date were implemented. The snowball method was then employed by reviewing reference lists of included articles for any additional relevant primary articles.
Results
Complications
TFESI are very low risk procedures; however, rare but serious
side effects can occur. Since the FDA investigation into neurological
complications, several studies have retrospectively investigated
complications arising from TFESI. One such retrospective cohort
study used HIRA claims to find patients who underwent TFESI
from 2009 to 2014 and subsequently presented to the emergency
department within 24 hours of the procedure. 830,000 cases were
found and the incidence of neurological complications, including
infections, hypotension, seizure, stroke, spinal cord injury, and
death, for PS versus NPS was 1.73 and 0.9 per 100,000, respectively.
Interestingly, neurological complication rates did not differ between
NPS and non-steroid injections [6]. A larger study by Scanlon, et
al. [13] aimed to determine the prevalence of neurological injuries
within the current population. A survey was sent to all physician
members of the American Pain Society. Respondents were asked
about awareness of complications, year, practice setting, use of
imaging, contrast, type of steroid, doses administered, and advanced
imaging findings. 287 respondents reported 78 complications
including 16 vertebrobasilar brain infarcts and 12 cervical spinal
infarcts. 13 of the 78 cases were fatal. Methylprednisolone was
used in 79% (22 of 28) of cases, betamethasone in 11% (3 of
28), and triamcinolone in 11% (3 of 28). Dexamethasone use was
not reported in any of the complications. The majority of serious
complications were due to infarcts; however, there was 1 case of
both air emboli and vascular dissection [13].
In addition to these retrospective studies, there are multiple
case reports of neurological events after TFESI [12,15,16]. To
date, there are only 2 case reports of complications associated
with NPS. The first was reported by Boudier-Revéret [11], where a 39-year-old man presented with involuntary movements of the
bilateral upper trapezius muscles after a right C6/7 TFESI with 5
mg dexamethasone. MRI of the brain and cervical spine revealed no
specific lesion and electroencephalography showed no abnormal
findings. The myoclonus gradually improved and resolved
completely within two weeks. It was difficult to discern the root
cause of the myoclonus; however, it was thought to be from trauma
leading to subacute spinal neuritis during neuraxial anesthesia.
The second case reported acute right lower extremity weakness
accompanied by a right-sided foot drop and sphincter dysfunction
after a dexamethasone right L5 TFESI [12]. MRI showed significant
disc herniations at the L4-5 and L5-S1 levels, contributing to
moderate central/foraminal stenosis but no acute findings. EMG
demonstrated acute denervation potentials in L5-S1 distributions.
Emergent L4-5, L5-S1 laminectomy with discectomies at the L4-5
and L5-S1 levels was performed. Immediately after surgery, the
patient’s weakness and sensory deficits improved. The authors
attributed this patient’s neurological deficit to an acute increase in
mass effect from the volume of injectate, resulting in ischemia.
Efficacy
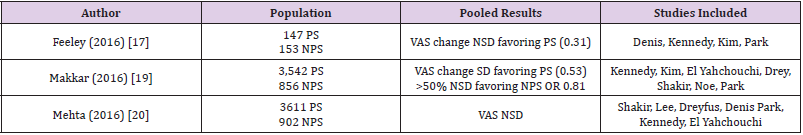
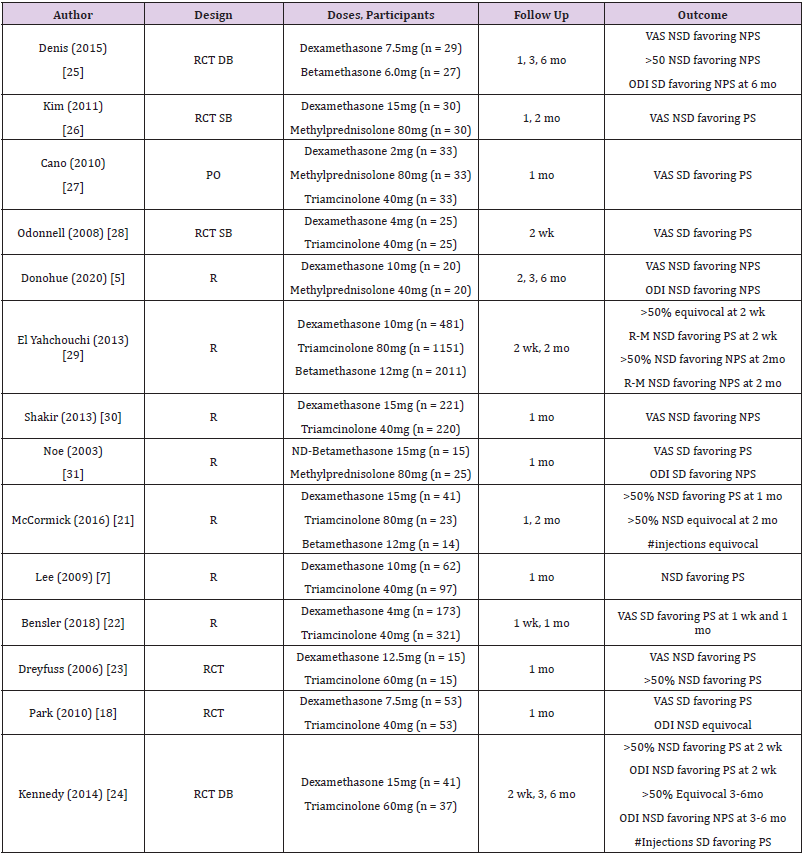
The efficacy of NPS versus PS for TFESI continues to be an area of controversy. An extensive evaluation of the literature shows multiple studies comparing the two. One large literature review conducted by Feeley and colleagues found four compatible studies comprising 300 participants undergoing 147 PS injections and 153 NPS injections [17]. Analysis showed no statistically significant difference in VAS data between the PS and NPS groups (0.31). There was however, a slight non-significant VAS improvement in those receiving PS injections. This review did include one study, Park, et al. [18], that showed a significant difference in VAS favoring PS over NPS injections. A larger systematic review and meta-analysis by Makkar et al. included studies that compared the efficacy of PS with NPS in TFESI [19]. Seven studies met criteria and included 3,542 PS and 856 NPS injections. Pooled average VAS scores were significantly different with 0.53-point greater VAS change for those receiving PS compared to NPS injections. However, the NPS group had a larger proportion of patients with more than 50% pain relief (OR 0.81). Once again, Park, et al. [18] was included in this review as the only study that reported a statistically significant difference in VAS scores between PS and NPS injections. A third meta-analysis by Mehta et al. also reviewed studies comparing 3611 PS and 902 NPS in TFESI [20]. Here, TFESI results were evaluated in 7 separate studies and again showed no significant difference in VAS scores between the two groups. Table 1 shows a summary of the 3 large systematic review studies and their results. While each review analyzed a different pool of studies, there was a fair amount of overlap. There were a few studies that were not included in any of the above analyses. These included McCormick et al., which studied 78 patients with three separate steroids and found no significant difference in the number of repeat injections required for pain relief, but slightly favored particulates for short-term pain relief [21].
Table 1: Systematic reviews comparing particulate and non-particulate steroids.
Note: NSD = No significant difference, SD = Significant difference
Greater than 50% relief was seen in 35% of those receiving
PS and 28% of those receiving NPS injections less than one month
after injections (<1 mo). Greater than 50% pain relief was seen in
40% of those receiving PS versus 39% receiving NPS injections
greater than one month following the injections (>1mo) [21]. A
second retrospective self-controlled study completed by Donohue
et al. included 40 patients receiving 20 PS and 20 NPS injections.
Non-particulates were favored when looking at both VAS score
and function outcomes [5]. Additionally, Lee et al. included 159
patients (97 receiving PS, 62 receiving NPS) who underwent
cervical TFESI and evaluated pain scores at 1 month. Outcomes
were similar to other studies, showing relief with PS in 80.4% of
patients versus 69.4% with NPS, although this difference was not
statistically significant [7]. There have been a limited number of
studies that have demonstrated a significant benefit of particulate
over non-particulate steroids for TFESI. These include Bensler, et
al. [22-24,18]. Bensler et al. performed a retrospective comparative
review on two cohorts in which one group (N=321) was treated
with 40mg triamcinolone and the other group (N=173) received
4 mg dexamethasone. PGIC scoring showed a significantly higher
proportion of patients improved at 1 week (43.2% versus 27.7%)
and one month (44.3% versus 33.1%) with PS over NPS [22].
Dreyfuss et al. completed a study involving 30 patients (15 PS, 15
NPS) and found the effectiveness of dexamethasone 12.5mg to be non-significantly less than that of triamcinolone 60mg at 1 month.
However, a greater proportion of the dexamethasone group (27%)
obtained complete relief of their pain than in the triamcinolone
group (7%), but this difference was not statistically significant
[23]. Another RCT that favored PS over NPS was completed by Park
and colleagues in which 106 patients underwent TFESI (53 NPS
7.5mg dexamethasone, 53 PS 40mg triamcinolone) and showed
significant improvement in the particulate group at 1 month [18].
For the dexamethasone group, the reduction of pain score was 40%
whereas that of the triamcinolone group was 71%.
A final multicenter double-blind RCT of 78 consecutive subjects
was completed by Kennedy, et al [24]. Looking at those receiving
triamcinolone (n=37) and dexamethasone (n=41), this study found
a greater percentage of PS subjects achieved ≥50% pain relief at 2
weeks than those receiving NPS (43.2 vs 31.7%); however, this did
not reach statistical significance and this difference disappeared
by the 3-month and 6-month follow-up. There was, however, a
statistically significant difference in the number of injections
received, with 17.1% of the dexamethasone group receiving three
injections vs only 2.7% of the triamcinolone group [24]. Table 2
shows a summary of all the available studies comparing the efficacy
of PS and NPS.
Table 2: Trials comparing efficacy of particulate and non-particulate steroids.
Note: RCT = Randomized control trial
DB = Double blind
SB = Single blind
PO = Prospective observational
R = Retrospective
ND = Non-depot
NSD = No significant difference
SD = Significant difference
Discussion
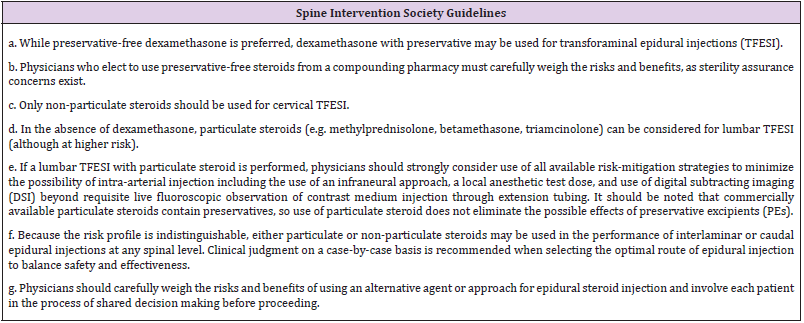
Intrinsically, there are risks and complications associated with any procedure. However, the focus of this article is to determine if there is added risk dependent on the type of steroid medication utilized in TFESI. Specifically, there is the generalized potential for air emboli, vasospasm and dissections, though these are very rare. The incidence of particulate emboli is rare but devastating when it occurs. There are numerous reports of PS-related emboli resulting in complications from paralysis to stroke or death. Catastrophic complications associated with NPS have not been reported. This catastrophic neurological risk associated with PS comes with the potential for a slight non-statistically significant pain reduction in the short term. Most studies that favor PS only followed patients for 1 month from injection. Studies that followed for a longer duration found that the difference between PS and NPS VAS scores disappeared. Bensler, et al. [22] showed a significant difference in favor of particulates, but the dosing used was not comparable given that 40mg triamcinolone was compared to 4mg dexamethasone (rough equivalent of 20mg triamcinolone). Given the body of evidence indicating increased risk and lack of compelling evidence of superior efficacy with PS, combined with the absence of any reported devastating complications attributed to NPS, it seems logical to advocate for the use of NPS for transforaminal epidural steroid injections. This is reiterated by the SIS guidelines posted in 2019 advising the use of NPS over PS whenever possible and directing physicians to advise patients of the increased risk with PS (Table 3) [25-31].
Conclusion
Though there is a strong preference among interventional pain physicians to utilize PS for TFESI, evidence strongly indicates that catastrophic complications, though rare, are exclusively associated with PS with no such reports with NPS. Furthermore, there is no compelling evidence of superior efficacy of PS over NPS other than minor, non-statistically significant early differences in VAS score that disappear with longer follow up periods. With the addition of the 2019 SIS Position Statement regarding the use of NPS for TFESI, it will be a growing challenge to justify the use of PS for TFESI given the increased risk. A shift to NPS for TFESI eliminates the likelihood of catastrophic neurological outcomes and is in line with the growing body of evidence and professional society guidelines. While position statements are non-binding, it is certainly possible that these types of documents may be relied upon for medicolegal purposes, thus creating the potential for liability for non-adherence.
References
- Benzon HT, Chew TL, McCarthy RJ, Benzon HA, Walega DR (2007) Comparison of the particle sizes of different steroids and the effect of dilution: a review of the relative neurotoxicities of the steroids. Anesthesiology 106(2): 331-338.
- Dietrich TJ, Sutter R, Froehlich JM, Pfirrmann CWA (2015) Particulate versus non-particulate steroids for lumbar transforaminal or interlaminar epidural steroid injections: an update. Skeletal Radiol 44(2): 149-155.
- Derby R, Lee SH, Date ES, Lee JH, Lee CH (2008) Size and aggregation of corticosteroids used for epidural injections. Pain Med 9(2): 227-234.
- (2016) FDA. FDA requires label changes to warn of rare but serious neurologic problems after epidural corticosteroid injections for pain. FDA Drug Safety Communication, US.
- Donohue NK, Tarima SS, Durand MJ, Wu H (2020) Comparing pain relief and functional improvement between methylprednisolone and dexamethasone lumbosacral transforaminal epidural steroid injections: a self-controlled study. Korean J Pain 33(2): 192-198.
- Hwang B, Lee J, Park BJ (2020) Neurological complication rates of epidural injections and selective nerve blocks: a comparison of steroid use patterns. Clin J Pain 36(6): 449-457.
- Lee JW, Park KW, Chung SK, Jin S Yeom, Ki Jeong Kim, et al. (2009) Cervical transforaminal epidural steroid injection for the management of cervical radiculopathy: a comparative study of particulate versus non-particulate steroids. Skeletal Radiol 38(11): 1077-1082.
- Manchikanti L, Hirsch JA (2015) Neurological complications associated with epidural steroid injections. Curr Pain Headache Rep 19(5): 482.
- Fitzgibbon DR, Posner KL, Domino KB, Robert A Caplan, Lorri A Lee, et al. (2004) Chronic pain management: American Society of Anesthesiologists closed claims project. Anesthesiology 100(1): 98-105.
- Wallace MA, Fukui MB, Williams RL, Ku A, Baghai P (2007) Complications of cervical selective nerve root blocks performed with fluoroscopic guidance. AJR Am J Roentgenol 188(5): 1218-1221.
- Boudier Reveret M, Chang MC (2020) Segmental spinal myoclonus after a cervical transforaminal epidural steroid injection. Am J Phys Med Rehabil 99(11): e128-130.
- Ghaly RF, Zouki T, Pynadath A, Candido KD, Knezevic NN (2018) Transforaminal epidural steroid injection can result in further neurological injury in a patient with severe foraminal stenosis and nerve impingement. Surg Neurol Int 9(1): 159.
- Scanlon GC, Moeller Bertram T, Romanowsky SM, Wallace MS (2007) Cervical transforaminal epidural steroid injections: more dangerous than we think? Spine (Phila Pa 1976) 32(11): 1249-1256.
- Belinda Duszynski (2019) Position statement on best practices for epidural steroid injections in the setting of a preservative-free dexamethasone shortage. Spine Intervention Society 20(7): 1277-1280.
- Jeon SH, Jang W, Kim SH, Cho YH, Lee HS, et al. (2021) Paraplegia after transforaminal epidural steroid injection in a patient with severe lumbar disk herniation – a case report. Anesth Pain Med (Seoul) 16(1): 96-102.
- Moon J, Kwon HM (2017) Spinal cord infarction after cervical transforaminal epidural steroid injection: case report and literature review. Case Rep Neurol 9(1): 1-5.
- Feeley IH, Healy EF, Noel J, Kiely PJ, Murphy TM (2017) Particulate and non-particulate steroids in spinal epidurals: a systematic review and meta-analysis. Eur Spine J 26(2): 336-344.
- Park CH, Lee SH, Kim BI (2010) Comparison of the effectiveness of lumbar transforaminal epidural injection with particulate and nonparticulate corticosteroids in lumbar radiating pain. Pain Med 11(11): 1654-1658.
- Makkar JK, Singh PM, Jain D, Goudra B (2016) Particulate vs non-particulate steroids for transforaminal epidural steroid injections: systematic review and meta-analysis of the current literature. Pain Physician 19(6): 327-340.
- Mehta P, Syrop I, Singh JR, Kirschner J (2017) Systematic review of the efficacy of particulate versus nonparticulate corticosteroids in epidural injections. PM R 9(5): 502-512.
- McCormick ZL, Cushman D, Marshall B, Mary Caldwell, Jaymin Patel, et al. (2016) Pain reduction and repeat injections after transforaminal epidural injection with particulate versus nonparticulate steroid for the treatment of chronic painful lumbosacral radiculopathy. PMR 8(11): 1039-1045.
- Bensler S, Sutter R, Pfirrmann CWA, Peterson CK (2018) Particulate versus non-particulate corticosteroids for transforaminal nerve root blocks: comparison of outcomes in 494 patients with lumbar radiculopathy. Eur Radiol 28(3): 946-952.
- Dreyfuss P, Baker R, Bogduk N (2006) Comparative effectiveness of cervical transforaminal injections with particulate and nonparticulate corticosteroid preparations for cervical radicular pain. Pain Med 7(3): 237-242.
- Kennedy DJ, Plastaras C, Casey E, Christopher J Visco, Joshua D Rittenberg, et al. (2014) Comparative effectiveness of lumbar transforaminal epidural steroid injections with particulate versus nonparticulate corticosteroids for lumbar radicular pain due to intervertebral disc herniation: a prospective, randomized, double-blind trial. Pain Med 15(4): 548-555.
- Denis I, Claveau G, Filiatrault M, Fugere F, Fortin L (2015) Randomized double-blind controlled trial comparing the effectiveness of lumbar transforaminal epidural injections of particulate and nonparticulate corticosteroids for lumbosacral radicular pain. Pain Med 16(9): 1697-1708.
- Kim D, Brown J (2011) Efficacy and safety of lumbar epidural dexamethasone versus methylprednisolone in the treatment of lumbar radiculopathy: a comparison of soluble versus particulate steroids. Clin J Pain 27(6): 518-522.
- Cano WG (2010) Is the particulate effect real?: comparison of the effectiveness, side effects and complication rate of the low particulate steroid dexamethasone vs. two high particulate steroids, triamcinolone and methylprednisolone when used in lumbar epidural injections. Pain Med 11(10): 1577-1578.
- Odonnell C, Cano W, Deramo G (2008) Comparison of triamcinolone to dexamethasone in the treatment of low back and leg pain via lumbar transforaminal epidural steroid injection. Spine J 8(5): 655.
- El Yahchouchi C, Geske JR, Carter RE, Felix E Diehn, John T Wald, et al. (2013) The noninferiority of the nonparticulate steroid dexamethasone vs the particulate steroids betamethasone and triamcinolone in lumbar transforaminal epidural steroid injections. Pain Med 14(11): 1650-1657.
- Shakir A, Ma V, Mehta B (2013) Comparison of pain score reduction using triamcinolone vs. dexamethasone in cervical transforaminal epidural steroid injections. Am J Phys Med Rehabil 92(9): 768-775.
- Noe CE, Haynsworth RF (2003) Comparison of epidural Depo-Medrol vs. aqueous betamethasone in patients with low back pain. Pain Pract 3(3): 222-225.

 Research Article
Research Article