Abstract
The current study is part of the development of medicinal plants known for their therapeutic virtues. We were then interested in studying sweet lime (citrus limetta) known for its richness in different compounds characterized by their important biological properties. The present work focused on extracting the peel of sweet lime by the maceration technique. The determination of phenolic compounds (hexane: 2.57 ± 0.0198; ethanol 150 ± 3.4; aqueous: 164.64 ± 2.37 mg equivalent in mg GAE/g DM), and in flavonoids (hexane: 1.544 ± 0.045; ethanol 64.991 ± 1.61; aqueous: 75.915 ± 0.65 mg equivalent mg QE/ g DM). Antioxidant activities were studied by direct, reactive oxygen species assay using chemical systems (TAA, DPPH, ABTS+, FRAP). The antioxidant effect on lipid peroxidation was studied in the biological system (cell culture), by measuring the levels of MDA and DC in HeLa cell line after H2O2 treatment. Our results showed protection against lipid peroxidation, highlighted by a significant decrease in MDA and DC levels (p <0.5 and p <0.01, respectively). The results showed a cytotoxic effect of the hexane extract on HeLa cell lines. In addition, the histological study on the skin fragments showed dose-dependent protection of our extracts against lesions caused by hydrogen peroxide.
Keywords: Citrus limetta; HeLa; Skin Fragments; Cytotoxicity
Introduction
Herbal medicines have formed the basis of traditional medical
systems for centuries and occupy an interesting place in modern
pharmacology. The World Health Organization (WHO) estimates
that about 80% of the world’s population still depends on herbal
medicines to treat various diseases because of their high availability
and economic reasons [1]. Citrus, belonging to the Rutaceae
family, is one of the most widely grown crops globally, especially
in tropical climates and temperate regions [2]. The health benefits
of citrus have been mainly associated with its high contents on
antioxidant molecules such as Vitamin C and Polyphenols known
for their benefic impact on human health [3]. Citrus belonged to
the family Rutaceae and several commercial citrus varieties such
as sweet orange, grapefruit, lime, and lemon and were considered
to possess natural compounds with several health benefits [4]. The
beneficial effects of citrus fruits can be attributed to their chemical
constituents, including vitamins, dietary fiber, carotenoids,
flavonoids, lipids, and essential oils [5,6].
Citrus limetta belongs to a type of citrus commonly known
as sweet lemon, which can be cultivated in South and Southeast
Asia [7]. It is small, between oval and spherical, with a greenishyellow
peel, rich in polyphenols, flavonoids, flavanones, and
flavones [8]. Citrus limetta Risso (Rutaceae) is known to be an
antihyperglycemic plant in Mexican folklore [9]. C. limetta fruits
are used for the common cold, decreasing cholesterol level, fever
regulation, regulating inflammation, digestive disorders, and so
forth as well as blood pressure modulator [10,11]. Flavonoids
hesperidin and naringin are found to be present in the peel part of
the fruit of Citrus limetta [12]. C. limetta comprised of 8–10% peel,
which is perishable waste material creates a challenge in processing
industries and pollution monitoring agencies [13]. Interestingly, C.
limetta fruit peels possess various pharmacological activities due to
flavonoids in a significant quantity [14,15].
In this context that we proposed to study in a first part the
extraction of fruit peels from the Citrus limetta plant by maceration
using three solvents (hexane, water and ethanol). In the second
part, we studied the content of total polyphenols and flavonoids and
the antioxidant effect. First, the antioxidant power was studied in a
chemical system (TAA, DPPH, ABTS, and FRAP). Then, the biological
system used HeLa cell lines by measuring MDA and CD levels. In
addition, to study the cytotoxic effect, MTT test was realized using
HeLa cells lines. Finally, the antioxidant activity of the three extracts
of Citrus limetta was evaluated on skin explants.
Materials and Methods
Plant Materials and Extraction Procedure
The Citrus limetta fruits were purchased from the local market / Sfax, south of Tunisia, in March 2019. The fruits were carefully washed, cutes into small pieces, and dried in an oven at 60 °C. 100 g of plant material were treated overnight with different solvents (ethanol, hexane, and water) under gentile stirring. The different extracts were filtered through a cellulose filter, lyophilized, and frozen at -80 °C until use.
Total Phenol Determination
Total phenols were determined using the Folin- Ciocalteu reagent according to the method of Singleton and Rossi [16]. Briefly, 50 μl aliquot of the extract was mixed with 250 μl of Folin reagent and 500 μl of sodium carbonate (20%, w/v). The mixture was vortexed and diluted with water to a final volume of 5 ml. After incubation for 30 min at room temperature, the absorbance was read at 765 nm. Total phenols were expressed as gallic acid equivalents (GAE), using a freshly prepared gallic acid solution calibration curve.
Total Flavonoid Determination
Total flavonoids were measured by the colorimetric assay described by Zhishen, et al. [17]. Total flavonoids were expressed on a dry weight basis as quercetin equivalents, using a freshly prepared quercetin solution calibration curve.
Antioxidant Capacity Assays
a. Phosphomolybdenum Assay
The total antioxidant activity of the extracts was evaluated using the Prieto, et al. methodology [18]. Briefly, 100 μl of the extract was mixed with 1 ml of the phosphomolybdenum reagent (600 mM sulfuric acid, 28 mM sodium phosphate, 4 mM ammonium molybdate). After incubation at 95 °C for 90 min, the absorbance was measured at 695 nm. The total antioxidant activity was expected to be the effective concentration (EC50) compared to Vit C as a positive control.
b. 2,2-Diphenyl− 1-picrylhydrazyl (DPPH) Free Radical Scavenging Activity Assay
The radical scavenging activity of extracts against DPPH free radicals was measured using the method of Cao, et al. [19] slightly modified as follows: 20 μl of appropriately diluted samples or Vitamin C solutions was added to 190 μl of DPPH solution (100 μM). The mixture was shaken vigorously and allowed to reach a steady-state at room temperature for 30 min. Then, the absorbance was measured at 517 nm with a Beckman spectrophotometer.
c. ABTS Radical Scavenging Activity Assay
The antiradical activity was performed by the ABTS+ free radical decolorization assay as developed by Re, et al. [20]. The reaction mixtures containing 100 μl of the sample at different concentrations and 900 μl of reagent were incubated at 30 °C for 6 min. The absorbance was measured at 734 nm.
d. Ferric-Reducing Antioxidant Power (FRAP)
The principle of this method is based on the reduction of a ferric-tripyridyltriazine complex to its ferrous, colored form in the presence of antioxidants [21]. Briefly, the FRAP reagent contained 2.5 ml of a 10 mmol/L TPTZ (2,4,6- tripyridy-s-triazine, Sigma) solution in 40 mmol/L HCl plus 2.5 ml of 20 mmol/L FeCl3 and 25 ml of 0.3 mol/L acetate buffer, pH 3.6 and was prepared freshly and warmed at 37°C. Aliquots of 40 μl sample supernatant were mixed with 0.2 ml distilled water and 1.8 ml FRAP reagent. The absorbance of the reaction mixture at 593 nm was measured spectrophotometrically after incubation at 37°C for 10 min. The 1 mmol/L FeSO4 was used as the standard solution. A spectrophotometer performs the absorbance reading against a blank at 700 nm. Ascorbic acid is used as a positive control. The increase in the absorption capacity of the components indicates an increase in iron reduction.
e. MTT Cell Proliferation Assay
MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell proliferation assay measures the cell proliferation rate and, conversely, reduces cell proliferation viability when metabolic events lead to apoptosis or necrosis. The yellow compound MTT (Sigma) is reduced by mitochondrial succinate deshydrogenase to the blue formazan, depending on the cell viability. Cells were grown on microtiter plates (200 μl of cell suspension/well) in 96 well microplates with serial extract dilutions. After 72 h, 20 μl of MTT solution (5 mg/ml) was added to each well. The plate was incubated for 4 h at 37 °C in a CO2 incubator. After that, 180 μl of the medium was removed from each well and replaced by 180 μl of (DMSO/ methanol) (V:V). When all the crystals were dissolved, absorbance was measured at 570 nm with a microplate reader (Elx 800 microplate reader) [22].
f. Malondialdehyde (MDA) Determination
The MDA production was evaluated using thiobarbituric acidreactive species (TBARs) assay. Adherent cells were detached using trypsin/EDTA solution and centrifuged at 3000 rpm for 10 min. The pellet was resuspended in 500 μl of deionized water and lysed by five cycles of sonication during 20 s at 35% (Sonisc, vibracell). One millilitre of TBA solution (15% trichloroacetic acid, 0.8% thiobarbituric acid, 0.25 N HCl) was added. The mixture was heated at 95 °C for 15 min to form MDA-(TBA)2 adducts. Optical density (OD) was measured by a spectrophotometer (Biochrom, Libra S32) at 532 nm. Values were reported to a calibration curve of 1,1,3,3-tetraethoxypropane (1.1.3.3 TEP) [23].
g. Conjugated Dienes
After sonication, cell lysates were extracted with 3 ml (chloroform: methanol) (2:1 v/v). After centrifugation at 3000 rpm for 15 min, 2 ml of the organic phase was transferred into another tube and dried at 45 °C. The dried lipids were dissolved in 2 ml of methanol, which was determined by absorbance at 233 nm. This corresponds to the maximum absorbance of the extracted compounds [24].
Evaluation of the Antioxidant Activity of Citrus Limetta Extract on Skin Explants
a. Stress Induction
After removal, the fragments are washed three times with PBS (1×) and then incubated for 15 min at room temperature in the presence of an antibiotic and an antifungal. The fragments are then sterile cut into small pieces and cultured in a 24-well plate at the air / liquid interface, in the presence and absence of the 3 extracts at different concentrations (0.5 mg / ml; 1 mg / ml; 2 mg / ml) for 2 hours at 37 °C. After incubation, the culture medium is eliminated, and then two successive washes with PBS (500 μl) are carried out. Hydrogen peroxide is then added at a concentration of 1.5 mM in RPMI to induce oxidative stress on the skin explants. After 2 h of incubation at 37 °C, all the fragments are recovered in tubes containing formalin and stored at 4 °C until the histological sections are made.
b. Histopathological Examination
For light microscopic examination, samples were fixed in 10% neutral buffered formalin, dehydrated in an ascending ethanol series (65%, 75%, and 95%), cleared in xylene, and embedded in paraffin. Paraffin sections (5 μm) were stained with hematoxylin and eosin using a routine method. The assembly step consists of bonding the blades containing the sections.
c. Analysis data
Statistical studies are carried out using the SPSS program (19.0). The t-Student test carried out the comparison between the means. The results are represented in mean ± standard deviation (DS).
Results
Total Phenolic and Flavonoid Compounds
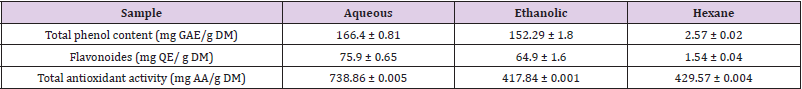
First, we were interested in evaluating the levels of anthocyanins, total phenolic, and flavonoïd compounds in the different Citrus limetta extracts using Follin-Ciocatleau colorimetric and AlCl3 methods, separately. The results show that the aqueous and ethanolic extract contains (166.4 ± 0.81 mg GAE / g DM; 152.29 ± 1.8 mg GAE / g DM) respectively. On the other hand, the hexane extract has a low polyphenol content (2.57 ± 0.02 EAG / g DM) (Table 1). Regarding flavonoids contents, our results indicate 75.9 ± 0.65 mg QE/g DM; 64.9 ± 1.64 mg QE/g DM for aqueous and ethanolic extract, respectively. In addition, a small number of flavonoids for hexane extract was detected (1.54 ± 0.045 EC / g DM) (Table 1).
Antioxidant Activity
a. Phosphomolybdenum Assay
The power of ammonium phosphomolybdate in the present study is expressed in mg equivalent of ascorbic acid (vitamin C) / g of dry matter. Our results indicate a total antioxidant activity for the Citrus limetta peels extracts (Table 1).
b. The Scavenging Activity for DPPH Radicals
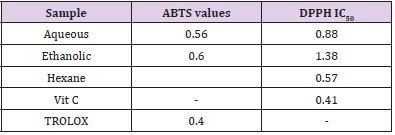
DPPH molecules that contain a stable free radical have widely been used to evaluate the radical scavenging ability of antioxidants. Therefore, the Citrus limetta free radical scavenging activities were assayed using DPPH (Table 2). At all the tested concentrations, Citrus limetta aqueous, ethanolic, and hexane extracts showed a significant anti-radicular effect attending 84%, 83%, and 87%, respectively, compared to their synthetic antioxidant positive control (BHT).
c. The Scavenging Activity for ABTS
The antioxidant activity test results of the ABTS+ radical by ethanolic, aqueous, and hexane extracts attend 89,9%; 90,10 and 8,24%, respectively (Table 2).
d. Ferric-Reducing Antioxidant Power (FRAP)
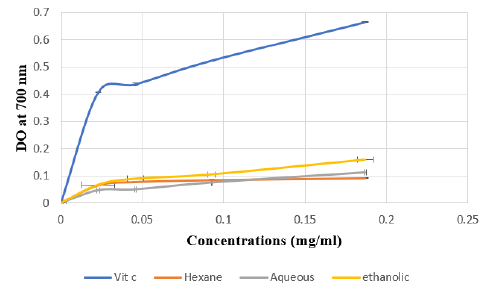
The FRAP assay is usually used to measure the capacity of the sample to reduce the ferric complex to the ferrous form. However, our results showed that the three Citrus limetta peel extracts have a weak antioxidant activity compared to vitamin C (Figure 1).
Figure 1: Absorbance of standard Vit C and extracts of Citrus limetta peel by Ferric Reducing Antioxidant Power (FRAP) assay.
Note: Various concentrations of extracts (0 to 0.25 mg/mL) and acid ascorbic were mixed with FRAP reagents. The reduction
of ferric ion (Fe3+) to ferrous form (Fe2+) by extracts produces an intense blue light revealed as a change in absorption at
700 nm. Results were expressed as mean inhibition percentage (%) ± standard deviations (n = 3). Ascorbic acid at various
concentrations (0 to 0.25 mg/mL) was used as standard.
e. Cytotoxicity Effect of Citrus limetta Peel Extract
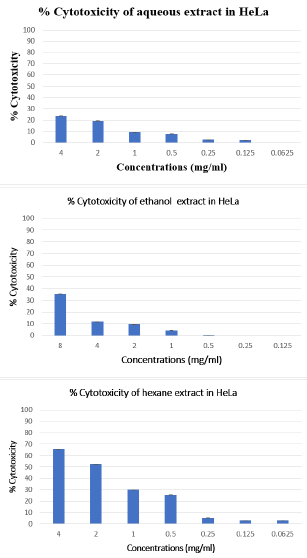
To investigate the cytotoxic effect of Citrus limetta peel extracts on HeLa human cell line, cells were treated with various concentrations of extracts (Hexane, ethanolic and aqueous) ranging from 4 to 0.0625 mg/ml for 48 hours, and then submitted to the MTT test. Our results showed a cytotoxic effect of the hexane extract on the HeLa line depending on the concentrations used. The other two extracts (Ethanol and Aqueous) have less than 50% cytotoxicity for the concentrations used (Figure 2).
Figure 2: Cytotoxic effect of Citrus limetta peel extracts on HeLa cell line.
Note: The inhibitory effect of different doses on cell growth was determined by MTT assay. Cells were treated with extracts at
a concentration ranging from 4 to 0.0625 mg/ml. The percent of growth reduction was calculated from the extinction difference
between treated cell culture and the control. Results are the means of two repetitions.
f. Effect of Citrus Limetta Peel Extracts on Lipid Peroxidation Activity
The investigation of the Citrus limetta extracts biological antioxidant activity was carried in HeLa human cell line. Cells were cultured with or without the addition of extracts for 48 hours. Oxidative stress was induced by adding 0,2 mM H2O2. Hydrogen peroxide and Citrus limetta extracts with a 0.25 mg/ml concentration were added to the HeLa human cell line simultaneously for 30 minutes.
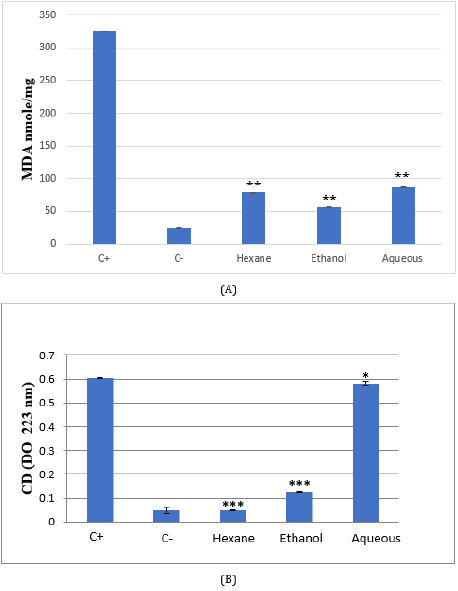
g. Malondialdehyde (MDA)
After H2O2 treatment, a very significant increase in MDA levels in the HeLa line was observed, in comparison with the untreated cells, indicating the presence of a state of oxidative stress following the treatment of these cells by H2O2 (p <0.01). HeLa cells treated with the three extracts: ethanol, hexane, and aqueous, induce a very significant decrease in MDA levels (p <0.01 respectively) (Figure 3).
h. Conjugated Dienes (CD)
HeLa cell line treated with H2O2 induces a significant increase in CD levels compared to untreated cells (p <0.001) (Figure 3). Conversely, treating cells with Citrus limetta extracts causes a significant decrease in CD levels. This decrease is highly significant for hexane and ethanol (p <0.001) and significant for the aqueous extract (p <0.05).
Figure 3: MDA (A) and conjugated diene (B) levels in Citrus limetta peel extracts supplemented HeLa cell line.
Cells were cultured in 25 cm2 flasks with 0.25 mg/ml of extracts for 48 hours. Oxidative stress was induced by the addition of
cells for 30 min. TBARs and conjugated diene (CD) were compared to untreated cells (C-), cells treated with H2O2 (C+), and
cells treated with extracts.
i. The Protective Effect on Skin Explants in Culture
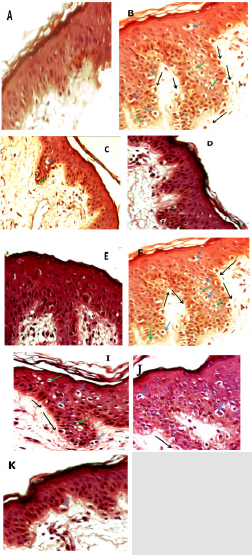
Figure 4: Micrograph of skin sections treated with the three extracts of Citrus limetta.
Note: A: control B: traited with H2O2 (1.5 mM) C: traitée with hexane extract (0.5 mg/ml) then with H2O2 (1.5 mM) D: traited
with hexane ectract (1 mg/ml) then H2O2 (1.5 mM) E: traited with hexane extract (2 mg/ml) then H2O2 (1.5 mM) F: traited with
aqueous extract (0.5 mg/ml) then H2O2 (1.5 mM) G: traited with aqueous extract (1 mg/ml) then H2O2 (1.5 mM) H: traited with
aqueous extract (2 mg/ml) then H2O2 (1.5 mM) I: traited with ethanolic extract (0,5 mg/ml) then H2O2 (1.5 mM) J : traited with
ethanolic extract (1 mg/ml) then H2O2 (1.5 mM) K : traited with ethanolic extract (2 mg/ml) then H2O2 (1.5 mM).
• Detachment of the basal lamina
• Pycnotic nucleus
• Vacuolar degeneration
The evaluation of the antioxidant activity of the three Citrus
limetta extracts was carried out on skin explants maintained
in culture in the presence of different concentrations of each
extract (0.5 mg/ml; 1 mg/ml; 2 mg/ml). The results obtained are
compared with those found with explants cultured in the presence
and absence of hydrogen peroxide (Figure 4). Our results have
shown that culturing of skin explants in the complete medium does
not affect the histo-architecture of the skin (Figure 4A). However,
treatment with hydrogen peroxide at a concentration of 1.5 mM
leads to structural disorganization of the skin, which manifests
itself in the detachment of the membrane which separates the
dermis and the epidermis. In addition, in the granular layer of
the epidermis, the cells presented a necrotic appearance which
resulted in retraction of the nuclei (pycnotic nuclei) and vacuolar
degeneration (Figure 4B).
The pretreatment of the skin explants at different concentrations
of each Citrus limetta peel extract made it possible to minimize the
oxidative damage induced by hydrogen peroxide depending on
the concentration used (Figures 4C-4N). It is observed that the
protective effect is most important at the level of the fragments
treated with the hexane extract (Figures 4C-4E), so the total
disappearance of the oxidative damage by the treatment with the
high concentration of hexane extract at 2 mg/ml.
Discussion
Plants contain different phenolic compounds, including simple
phenolics, phenolic acids, hydroxycinnamic acid derivatives and
flavonoids [25]. The content of total polyphenols and flavonoids
was determined. Our results agree with those of Mohanty, et al. [15]
who showed the content of phenolic and flavonoid compounds in
the aqueous and ethanolic extract of Citrus limetta [15]. Similarly,
Safdar et al showed that the 4 peel extracts of Citrus reticulate L
(methanols, ethanol, acetone, and ethyl acetone) presented a
polyphenol content varying according to the polarity of the solvents
used in the extraction [26]. Our results agree with the work of
Hegazy et al. [27] who found that the polyphenol content varies
between the different extracts of orange peel [27]. According to
Chan et al. the choice of extraction solvent is essential because it
allows the type and quantity of polyphenols to be estimated [28].
The variations in the polyphenol content depend on the polarities
of the solvents used and their concentration level.
Citrus limetta extracts analyzed in this work showed potent
radical scavenging activity. We have demonstrated that GEo has
an interesting antioxidant activity which is highlighted by the
TAC, DPPH, ABTS+, FRAP. Our results agree with the literature
that showed that citrus possesses an anti-free radical activity
DPPH, according to Padilla, et al. [29]. The Citrus limetta aqueous
extract can trap the DPPH radical with a percentage inhibition of
42.5%. Other Citrus reticulate L methanolic extract studies have
shown a high antioxidant potential (72.83 ± 1.22%) [26]. Other
work studied three extracts of the peel orange (Citrus aurantium)
that showed significant antioxidant power with low IC50 values
(acetone 781.9 μg / ml; ethanol 1,137.9 μg / ml; methanol 1,402.9
μg / ml) [30]. The same extract showed comparable antioxidant
activity to TROLOX using the ABTS radical. Our results agree with
Oboh et al., who showed the presence of an antioxidant effect of the
peel of Citrus sinensis, Citrus paradisii, and Citrus maxima extracts
against the radicals ABTS [31].
The Citrus limetta extracts had a weak FRAP value, which was
in disaccord with the results obtained by Farha et al. that confirmed
that the Citrus limetta methanolic extract has moderate antioxidant
(iron-reducing capacity) activity [32]. The variation in antioxidant
activity depending on the tests can be explained by the fact that this
antioxidant activity depends not only on the concentration but also
on the structure and antioxidants nature [33]. Our results showed a
cytotoxic effect-dependent concentration of Citrus limetta extracts
with Hexane on HeLa cell lines. Other studies on the aqueous
extract of Citrus unshiu (a species of the same family as the Citrus
limetta) showed the presence of a cytotoxic power of the latter
on the line MDA-MB-231 with a concentration of 1,5 mg/ml. This
cytotoxic effect is attributed to the disruption of the mitochondrial
transport system [34]. On the other hand, Diab et al. showed that
the ethanolic peel extracts of Citrus sinensis L reduced the HL-60
cell line viability [35].
According to the results, to investigate the antioxidant activity
of our extract on the cells model, we choose a concentration that
induces less than 20% of toxicity. The concentration used is 0.25
mg/ml in all the experiences. HeLa cells are treated simultaneously
with H2O2 and extracts. Our results induce a very significant
decrease in MDA levels, which agrees with the work, which
showed that the methanolic extract of Citrus limetta peel exhibits
antioxidant activity demonstrated by a reduction of MDA levels [36].
To the best of our knowledge, our study is the first to determine the
antioxidant potentials of Citrus limetta peel on the skin by analyzing
histopathological findings.
Conclusion
Citrus limetta is a promising source of natural antioxidants, as indicated by its high contents of polyphenols, flavonoids and by its considerable DPPH and ABTS free radical scavenging activities and FRAP value. In addition, the histological study of skin fragments has shown dose-dependent protection of our extracts against lesions.
Acknowledgment
This work is dedicated to the memory of Pr. Saloua Lassoued Elamri. We will never forget her, and she is always in our hearts.
References
- Milind P, Dev C (2012) Eat an Orange to Keep Anxiety At Long Range. International Research Journal of Pharmacy 3(10).
- Chutia M, Deka BP, Pathak MG, Sarma TC, Boruah P (2009) Antifungal activity and chemical composition of Citrus reticulata Blanco essential oil against phytopathogens from Northeast India. LWT - Food Science and Technology 42(3): 777-780.
- Ramful D, Tarnus E, Aruoma OI, Bourdon E, Bahorun T (2011) Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Research International 44(7): 2088-2099.
- Gyawali R, Kim S (2014) Anticancer Pytochemicals of Citrus Fruits. Journal of Animal Research 4(1): 85-96.
- Kurowska EM, Spence JD, Jordan J, Wetmore S, Freeman DJ, et al. (2000) HDL cholesterol raising effect of dietary orange juice in subjects with hypercholesterolemia. American Journal of Clinical Nutrition 72(5): 1095-1100.
- Pragasam SJ, Rasool M (2013) Dietary component p-coumaric acid suppresses monosodium urate crystal-induced inflammation in rats. Inflammation Research 62(5): 489-498.
- Hashemi SM, Khaneghah AM, Barba FJ, Nemati Z, Shokofti SS, et al. (2017) Fermented sweet lemon juice (Citrus limetta) using Lactobacillus plantarum LS5: Chemical composition, antioxidant and antibacterial activities. Journal of Functional Foods 38(A): 409-414.
- Kim G N, Shin J G, Jang HD (2009) Antioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoi. Food Chemistry 117(1): 35-41.
- Andrade Cetto A (1995) Estudio Etnobot´anico y Fitoqu´ımico de plantas ´utiles en la regi´on de Xochipala Guerrero para el tratamiento de la diabetes NID, M.S. thesis, Facultad de Ciencias, UNAM, Mexico (Ethnobotanical and Phytochemical Study of Useful Plants in the Xochipala Guerrero Region for the Treatment of Diabetes NID, M.S. thesis, Faculty of Sciences, UNAM, Mexico).
- Clement Y N, Morton Gittens J, Basdeo L, Blades A, Francis MJ, et al. (2007) Perceived efficacy of herbal remedies by users accessing primary healthcare in Trinidad. BMC Complementary and Alternative Medicine 7: 4.
- Argueta V A, Cano A L, Rodarte M E (1994) Citrus limetta, in Atlas de las Plantas de la Medicina Tradicional Mexicana, Instituto Nacional Indigenista, Eds., pp. 902-903.
- Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, et al. (2006) Flavonoid composition of fruit tissues of citrus species. Bioscience, Biotechnology and Biochemistry 70(1): 178-192.
- Manthey A, Grohmann K (2001) Phenols in citrus peel byproducts: concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agric Food Chem 49(7): 3268-3273.
- Bharti S, Rani N, Krishnamurthy B, Arya D S (2014) Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med 80(6): 437-451.
- Mohanty S, Maurya A K, Jyotshna, Saxena A, Shanker k, et al. (2015) Flavonoids rich fraction of Citrus limetta fruit peels reduces proinflammatory cytokine production and attenuates malaria pathogenesis. Curr Pharm Biotechnol 16(6): 544-552.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolic with phosphomolibdic phosphotungstic acid reagents. Am J Enol Vitic 16: 144-158.
- Zhishen J, Mengcheng T, Jianming W (1999) The determination of Flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64(4): 555-557.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry 269(2): 337-341.
- Cao L, Si J Y, Liu Y, Sun H, Jin W, et al. (2009) Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chemistry 115(3): 801-805.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, et al. (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radicals Biol Med 26(9-10): 1231-1237.
- Benzie I F F, Strain J J (1996) The ferric reducing ability of plasma as a measure of “antioxidant power:” the FRAP assay. Anal Biochem 239(1): 70-76.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65(1-2): 55-63.
- Gargouri B, Lassoued S, Ayadi W, Karray H, Masmoudi H, et al. (2009) Lipid peroxidation and antioxidant system in the tumor and in the blood of patients with nasopharyngeal carcinoma. Biological Trace Element Research 132(1-3): 27.
- Kurien B T, Scofield R H (2003) Free radical mediated peroxidative damage in systemic lupus erythematosus. Life Sciences 73(13): 1655-1666.
- Kumar N, Pruthi V (2014) Potential applications of ferulic acid from natural sources. Biotechnol Rep 4: 86-93.
- Safdar M N, Kausar T, Jabbar S, Mumtaz A, Ahad K, et al. (2017) Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. Journal of Food and Drug Analysis 25(3): 48-500.
- Hegazy A E, Ibrahium M I (2012) Antioxidant Activities of Orange Peel Extracts. World Applied Sciences Journal 18(5): 684-688.
- Chan S W, Lee C Y, Yap C F, Mustapha W A W, Ho CW (2009) Optimisation of extraction conditions for phenolic compounds from limau purut (Citrus hystrix) peels. International Food Research Journal 16(2): 203-213.
- Padilla C E, Lazcano D E, Flores F J M, Owolabi M S, Allen K, et al. (2014) Evaluation of the Inhibition of Carbohydrate Hydrolyzing Enzymes, the Antioxidant Activity, and the Polyphenolic Content of Citrus limetta Peel Extract. The Scientific World Journal.
- Park J H, Lee M, Park E (2014) Antioxidant Activity of Orange Flesh and Peel Extracted with Various Solvents. Preventive Nutrition and Food Science 19(4): 291-298.
- Oboh G, Ademosun A O (2012) Characterization of the antioxidant properties of phenolic extracts from some citrus peels. Journal of Food Science and Technology 49(6): 729-736.
- Farha S, Chatterjee E, Manuel S G A, Reddy S A, Kale R D (2012) Isolation and Characterization of Bioactive Compounds from Fruit Wastes. Dynamic Biochemistry, Process Biotechnology and Molecular Biology 2: 92-94.
- Falleh H, Ksouri R, Chaieb K, Karray B N, Trabelsi N, et al. (2008) Phenolic composition of Cynara cardunculus L. organs, and their biological activities Pharmacology, toxicology. C. R. Biologies 331(5): 372-379.
- Kim M Y, Choi E O, HwangBo H, Kwon D H, Ahn K I, et al. (2018) Reactive oxygen species-dependent apoptosis induction by water extract of Citrus unshiu peel in MDA-MB-231 human breast carcinoma cells. Nutrition Research and Practice 12(2): 129-134.
- Diab K A E, Shafik R E, Yasuda S (2015) In Vitro Antioxidant and Antiproliferative Activities of Novel Orange Peel Extract and It’s Fractions on Leukemia HL-60 Cells. Asian Pacific Journal of Cancer Prevention 16(16): 7053-7060.
- Kundu Sen S, Saha P, Bhattacharya S, Bala A, Gupta M, et al. (2010) Evaluation of in vitro antioxidant activity of citrus limetta and citrus maxima on reactive oxygen and nitroge species. Pharmacologyonline 3: 850-857.

 Research Article
Research Article