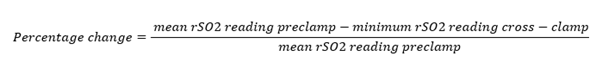ABSTRACT
Background: The aim of this pilot investigation was to see if a raised serum S100B level or a decrease in rSO2 following carotid revascularization with CEA might be used to detect neurological instability in CEA patients. Increased serum S100B levels during CEA, we hypothesized, would be linked to neurological symptoms after surgery. Patients and methods: A total of 64 consecutive CEAs in 60 patients operated under LA (local anesthesia) during an 18-month period were prospectively evaluated. The cerebral oximeter was used to measure cerebral oxygen saturation (rSO2) before and after cross-clamping along with serum. concentration of S100B protein. Selective shunting was performed when neurological changes occurred, regardless of NIRS.
Results: The neurological symptoms that occurred after clamping correlated with an increase in the serum level of S100B (P = .040). The cut-off of 22.5% of S100B increase was determined to be optimal for identifying patients with neurological symptoms. There was no correlation between rSO2 decline and neurological symptoms (P = .675). Two (3.1%) perioperative strokes occurred.
Conclusions: Awake neuromonitoring has been found to provide a sensitive and direct evaluation of brain tissue perfusion and is specific to CEA under LA. Although there was a favourable connection between CEA and an increase in serum S100B protein, due to the long assessment time, serum S100B monitoring was not practicable (usually 3 hours).
Keywords: Perioperative Stroke Prevention; Neuromonitoring; Carotid Stenosis; Selective Shunting
Introduction
Carotid Endarterectomy (CE) is a surgical procedure that has been widely accepted as the appropriate means to reduce the likelihood of Ischaemic Stroke (IS) in the setting of severe carotid artery stenosis. A possible complication of the surgery may be IS. The main cause of a cerebrovascular insult is reduced blood flow through the brain during cross clamping of the Internal Carotid Artery (ICA). Immediate and correct recognition of insufficient collateral flow is crucial for a good outcome of the vascular surgery [1]. General use of a temporary vascular bypass (shunt) during surgery increases the complexity of the endarterectomy and may injure the vascular wall, leading to thromboembolism [2]. This implies the need for neuromonitoring to select patients who will benefit from shunt insertion [3]. Patients undergoing CEA under Local Anaesthesia (LA) are monitored clinically, preferably with neuromonitoring, which enables a more appropriate shunt insertion than other methods [4].
This is not possible in patients undergoing surgery in general anaesthesia, and therefore a suitable method of good neurological monitoring is being sought for these patients. Objective value of methods of monitoring patients during CEA was evaluated by studying serum levels of biochemical markers of brain injury. One of the most investigated is the serum protein S100B [5]. The neuroprotein S100B is a subunit of calcium-binding protein B identified in astroglia and Schwann cells. S100B’s function has yet to be fully explored and defined. At nanomolar concentrations, S100B stimulates axon development and neuronal survival. At micromolar levels, it causes apoptosis and increases the production of inflammatory cytokines [6]. Even during clinically atypical CEA, S100B in peripheral blood is a sensitive sign of Blood-Brain Barrier (BBB) failure and ischemic brain injury [7,8]. Peak S100 B levels can be found as early as 20 minutes after brain injury. On the other hand, it has a biological half-life of 30 to 113 minutes and is eliminated quickly by the kidneys [9]. In the previous decade, concerns regarding mild, subclinical brain harm after carotid revascularization have been raised [10].
Cappocia [11] et al. analyzed SB100 values as continuous and categorical data and found that they can be correlated with subclinical brain injury to some extent. Sahlein [12]. Inserted a 6 French Fogarty catheter rostral to the jugular bulbus through the facial vein. Fifteen minutes after carotid artery blockage, S100B levels in the jugular bulbus were considerably higher compared to baseline values. Some studies linked slight cerebral injuries following CEA and a large increase in serum S100B levels [13,14]. Several studies have found a large increase in S100B levels after declamping [15,16], followed by a reduction in S100B levels 24 hours later [10,17]. Falkensammer [18] investigated the relationship between perioperative levels of the molecular marker S100B and the likelihood of subclinical abnormalities in cerebral function and mild cerebral injury following CEA. Aleksic [4] observed a small but considerable increase in arterial S100B levels during carotid ventricular declamping.
NIRS (near-infrared spectroscopy) is a technique for determining regional oxygen saturation (rSO2). NIRS monitoring was employed in our department during CEA in all patients. A near-infrared light (NIR) emitting element is placed on the skin above the forehead to perform NIRS. Because NIR light penetrates through human tissue, some of it passing through the skin, subcutaneous tissue, and bone. The cerebral parenchyma reflects some of the light that reaches the brain. At particular wavelengths, the reflectance of brain tissue is substantially determined by the concentration of oxygenated haemoglobin in the tissue. The device records the amount of reflected photons as a function of wavelength by detecting the reflected photons. The INVOS system determines tissue oxygenation by measuring the amount of reflected light at wavelengths of 730 and 810 nm and calculating the regional oxygen saturation of haemoglobin (rSO2 index, sometimes known as simply rSO2) [19]. The aim of this pilot investigation was to see if a raised serum S100B level or a decrease in rSO2 following carotid revascularization with CEA might be used to detect neurological instability in CEA patients. Increased serum S100B levels during CEA, we hypothesized, would be linked to neurological symptoms after surgery.
Patients and Methods
Study Population
A prospective observational study design was performed with patients admitted to the Department of Vascular Surgery of Novo mesto General Hospital who underwent CEA between. Sixty adults (41 men, 19 women) between the ages of 50 and 86 years who underwent 64 CEAs over a 12-month time period were studied. The approval of the National Medical Ethics Committee of the Republic of Slovenia was obtained; written informed consent was obtained from all the patients. Indications for CEA included ipsilateral neurological symptoms (stroke, Transient Ischaemic Attack [TIA], amaurosis fugax), with ≥50% ICA stenosis, and both symptomatic and asymptomatic patients with 70% to 99% stenosis.
Carotid Endarterectomy
Twenty-six patients were symptomatic, and all were scheduled to undergo CEA with regional anaesthesia achieved by the combination of superficial and deep cervical plexus block (100 mg levobupivacaine + 200 mg lidocaine). After carotid clamping, neurological assessment was performed by having the patient squeeze into the contralateral hand and speak. Neurological assessment was continuous throughout the operative procedure at 3-min intervals. The patients were assigned to one of two groups: those who developed neurological symptoms (neurological symptoms group) during clamping and those who did not (no neurological symptoms group). Criteria for the neurological symptoms group were development of motor weakness, slurring of speech, inability to respond appropriately to verbal commands, loss of consciousness, or seizure. These were also used as criteria for insertion of a shunt or anticipating a very short clamp time with primary closure. The patch closure was performed in 58 CEAs, with primary closure in 6.
Cerebral Oximetry
The cerebral oximeter INVOS 5100C (Somanetics) was used to measure simultaneous, bilateral rSO2 throughout the procedure. During surgery, brief and variable degrees of cerebral ischaemia occur during cross-clamping of the ICA [20,21]. The pre-clamping bilateral rSO2 value and the lowest ipsilateral measurement after ICA clamp placement were recorded. Inter subject variability in rSO2 index values is well known and was noticed in this study. To facilitate comparison of rSO2 changes after carotid cross-clamp among all patients, and to determine the magnitude of rSO2 change that was associated with a change in neurological function, the rSO2 data were normalised by calculating a percentage change in rSO2 reading during cross-clamp periods in each patient according to a formula (Figure 1). On the basis of previous studies [22], a decrease in rSO2 of ≥12% was considered clinically significant.
Figure 1: Thus, a change of rSO2 reading from a mean preclamp value of 60% saturation to a minimal (after cross-clamp) of 54% saturation according to this formula would represent a 10% decrease in rSO2 reading (percentage change = 10%).
Serum Biomarker of Brain Injury
Venous blood samples were obtained for each patient preoperatively (basal sample, preclamp), immediately after the end of the procedure (declamp), and 12 hours, 24 hours, and 48 hours after the surgery. Samples were allowed to clot. Blood samples were centrifuged within 30 minutes, and serum was stored at –20°C until assayed in duplicate in a single batch within 6 months. The concentrations of protein S100B and NSE were measured by automated electrochemiluminescence assay (Cobas e411 analyser, Roche Diagnostics, Mannheim, Germany). The lower limit of detection for protein S100B was 0.005 μg/L. The upper reference limit of protein S100B was set at 0.105 μg/L, representing the 95th percentile of the healthy population. The reference limit was provided by the manufacturer of the assay and verified by the laboratory. We compared the baseline value with the sample taken immediately after the end of the procedure. An increase of 25% was considered abnormal [11].
Statistical Analysis
The lowest values of rSO2 and highest values of S100B were used for comparison between the no neurological symptoms and neurological symptoms groups. The baseline characteristics of patients who developed neurological symptoms were compared with the patients with no symptoms using the chi-square test or Fisher exact test, where appropriate. In the case of continuous variables, the independent samples t-test or Mann-Whitney test was used. Of these results Positive Predictive Value (PPV), Negative Predictive Value (NPV), positive Likelihood Ratio (LR+), Negative Likelihood Ratio (LR-) and Diagnostic Odds Ratio (DOR) with 95% Confidence Intervals (CI) were calculated. ROC curve analysis was applied to identify the threshold values of parameters and the Area Under the Curve (AUC) values were compared. Statistical analysis was performed with R 3.5.2 statistical software (R Foundation for Statistical Computing, Vienna, Austria). A p-value less than 0.05 was considered as statistically significant.
Results
Baseline Characteristics
In this study, 64 CEAs were performed under regional anaesthesia. There were 41 (67%) men and 19 (33%) women; patient mean age (±SD) was 70.9 ± 8.5 years (range, 50-86 years). Two men and two women were operated bilaterally. Fifty-nine per cent of the CEAs were performed for asymptomatic disease, in comparison with 41% for symptomatic disease.
Demographics, Anatomy, and Pre-Operative Variables
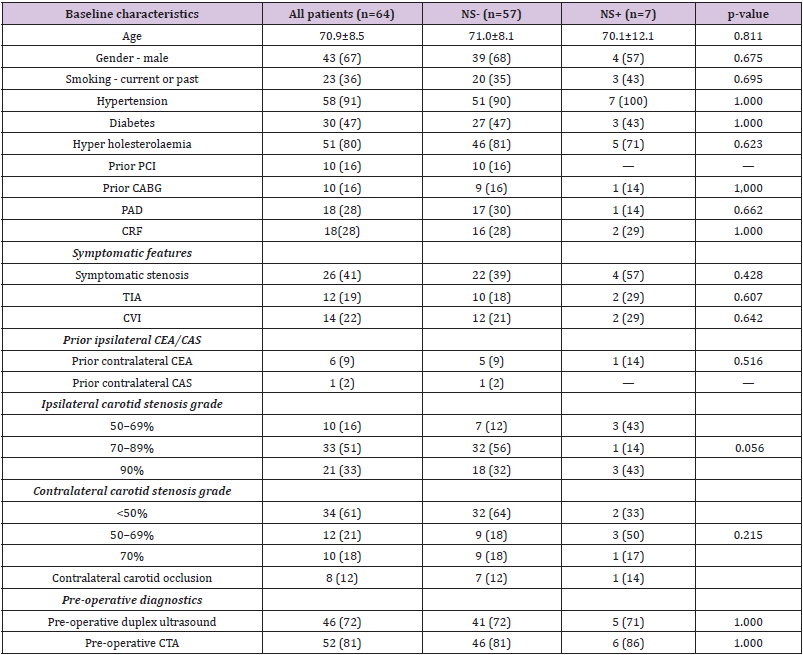
There were no differences (Table I) in diabetes mellitus, hypertension, smoking, hypercholesterolaemia, coronary artery bypass graft, and chronic renal failure or peripheral artery disease between those with and without neurological symptoms. There were no significant differences in rates of overall prior carotid intervention, symptomatic features, contralateral carotid stenosis grade, or pre-operative diagnostics between the two groups. However, ipsilateral carotid stenosis grade of 50–69% (43% vs. 12%) were more common in those with neurological symptoms and ipsilateral carotid stenosis grade of 70–89% (57% vs. 88%) were more common in those without neurological symptoms (P = .056).
Table 1: Baseline characteristics of no neurological symptoms (NS-) and neurological symptoms (NS+) group.
Note: Results are presented as n (%) or mean ± standard deviation.
PCI: Percutaneous Coronary Intervention; CABG: Coronary Artery Bypass Graft; PAD: Peripheral Arterial Disease; CRF: Chronic Renal Failure; TIA: Transient Ischaemic Attack; CVI: Cerebrovascular Insult; CEA: Carotid Endarterectomy; CAS: Carotid Artery Stenting; CTA: Computer Tomographic Angiography.
Operative and Post-Operative Variables
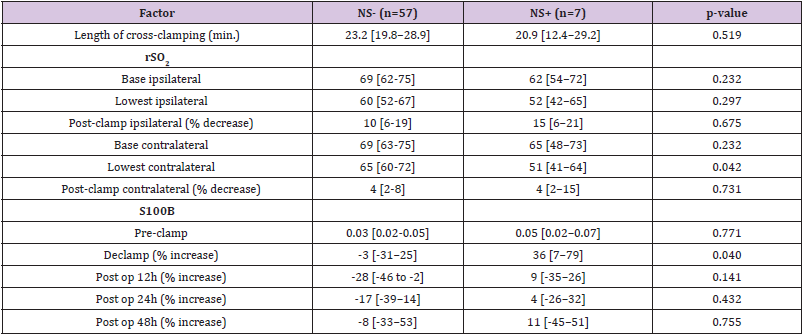
Neurological deterioration after carotid clamping occurred (neurological symptoms group) in 7 (10.9%) operations. Neurological change resolved after insertion of an intravascular shunt. The median [1stQ, 3rdQ] duration of carotid cross-clamping were 23.2 [19.8, 28.9] minutes and 20.9 [12.4, 29.2] minutes in the no neurological and neurological symptoms groups, respectively (Table 2). This difference was not statistically significant (P=.519). A prosthetic patch was used in 58 (90.6%) procedures and primary closure in 6 (9.4%) procedures.
S100B
Table 2: Operative in post-operative factors in patients without Neurological Symptoms(NS-) and patients with Neurological Symptoms (NS+) during CEA.
Note: Results are presented as median [interquartile range].
CEA = carotid endarterectomy; rSO2 = cerebral oxygen saturation; S100B = protein S100B.
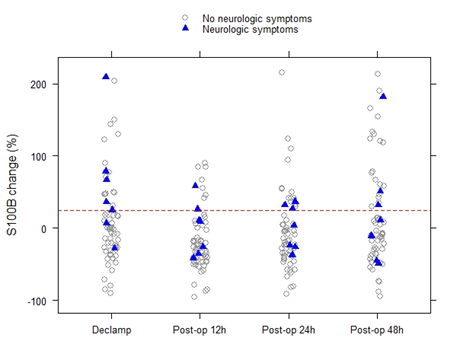
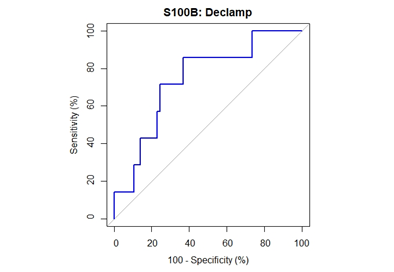
Median [1stQ, 3rdQ] baseline serum levels of protein S100B in the asymptomatic patients and symptomatic patients were 0.030 [0.021, 0.053] and 0.037 [0.025, 0.060], respectively. There were no significant differences in baseline preclamp concentrations of protein S100B between the patients (P = .456; Mann-Whitney U test). Percentage of increase in S100B parameter at different timeframes for the no neurological symptoms and neurological symptoms groups are depicted in Figure 2. The median serum S100B level increase was 36.2% [6.9%, 79.0%] (median [1stQ, 3rdQ]) in the neurological symptoms group, compared with –2.7% [–30.9%, 25.5%] (median [1stQ, 3rdQ]) in the no neurological symptoms group. The highest increase of serum S100B protein was 209%. The increase was significantly different between the groups (P = .040; Mann-Whitney U test) (Figure 2). This finding indicates that neurological instability that occurs after clamping correlates with the increase in S100B level. Neurological change could be predicted as a function of a 25% increase of S100B marker. Applying this technique, the Area Under the Curve (AUC) was 0.7393 (95% confidence interval CI = [0.5472, 0.9315]), and the diagnostic sensitivity and specificity were 71.4% and 75.4%, respectively. The threshold for S100B of 22.5% increase was optimal to identify patients with neurological symptoms (Figure 3 & Table 3).
Figure 2: Percentage of increase in S100B parameter at different timeframes for the no neurological symptoms and neurological symptoms groups.
Figure 3: ROC curve for performance of S100B declamp percentage change in prediction of neurological symptoms. The closest top left point is at threshold value 22.5% with sensitivity of 71.4% and specificity of 75.4%. The area under the curve is 74% (95% CI: 55-93%).
Cerebral Oxygen Saturation
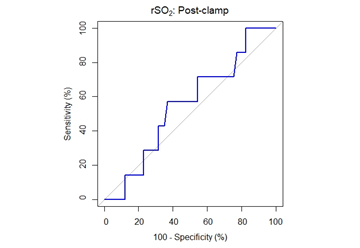
The decrease in rSO2 from the preclamp to cross-clamp period on the ipsilateral side was not statistically significant between the groups. The median rSO2 decrease was 15% [6%, 21%] (median [1stQ, 3rdQ]) in the neurological symptoms group vs. 10% [6%, 19%] (median [1stQ, 3rdQ]) in the no neurological symptoms group (P=.675, Mann-Whitney U-test). The correlation between changes in rSO2 and neurological symptoms was analysed and by ROC analysis, a cut-off of rSO2 decrease of 13.4% was determined to be optimal for identifying patients with neurological symptoms, AUC = 0.5489, 95% CI = [0.3327, 0.7651]. Sensitivity and specificity were 57.1% and 63.2%, respectively (Figure 4 & Table 3).
Table 3: Results of ROC curve analysis with threshold values.
Note: Results are presented as value (95% CI).
AUC: Area Under The Curve; rSO2: Cerebral Oxygen Saturation; S100B: Protein S100B.
Figure 4: ROC curve for performance of rSO2 post-clamp percentage change in prediction of neurological symptoms. The area under the curve is 55% (95% CI: 33-77%).
Contralateral Carotid Occlusion
There were no statistically significant differences between contralateral carotid occlusion and neurological symptoms (P=1), contralateral carotid occlusion and serum S100B increase (P=.466), or contralateral carotid occlusion and rSO2 fall (P=.418).
Prediction of Neurological Symptoms
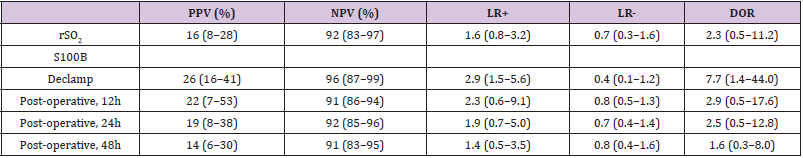
The PPV for the prediction of neurological symptoms during the CEA was 16% for the rSO2 parameter; for the S100B parameter the values were 26% for declamp, 22% for 12h post-operative, 19% for 24h post-operative, and 14% for 48h post-operative. The NPV for the rSO2 parameter and S100B parameter at different timeframes were 92%, 96%, 91%, 92% and 91%, respectively (Table 4). However, in terms of LR considered as minimally predictive, the declamp S100B parameter had amongst other measurements the highest LR+ (2.9, 95% CI: 1.5-5.6) and lowest LR- (0.4, 95% CI: 0.1- 1.2). Also in terms of DOR, this parameter performed best (7.7, 95% CI: 1.4-44.0).
Table 4: Performance of rSO2 and S100B parameters in prediction of neurological symptoms.
Note: Results are presented as value (95% CI).
rSO2: cerebral oxygen saturation; S100B: Protein S100B; PPV: Positive Predictive Value; NPV: Negative Predictive Value; LR+: Positive Likelihood Ratio; LR-: Negative Likelihood Ratio; DOR: Diagnostic Odds Ratio
Peri-Operative Outcomes
Two (3.1%) perioperative strokes occurred. The single major stroke happened on day 6, and the patient had decreased responsiveness to verbal commands and developed left hemiplegia. The diagnosis of stroke was based on the clinical and computed tomography finding of a focal ipsilateral ischaemic cerebral infarct with a patent CEA site on angiography. The patient was asymptomatic. The other patient had mild neurological deficits with complete recovery without re-exploration of the carotid artery. The patient was symptomatic. Both patients were shunted, and had normal rSO2, and increased S100B markers.
Discussion
The key conclusion of this pilot investigation was that increased serum levels of S100B protein, a brain marker, were associated with increased neurological instability. A statistically significant difference was only revealed by decapitation measurements and disregarded by 12-, 24-, and 48-hour post-operative measurements. The early mechanism of S100B release, which happens minutes later, is still unknown. The low amount of hypoperfusion associated with carotid artery occlusion may cause minor blood-brain barrier disruption, which could explain the early rise of serum S100B. [12,23]. Although S100B been linked to cerebral ischaemia, developing it into a biomarker for differential diagnosis in clinical practice has been problematic [24,25]. Several writers have claimed that elevated levels of S100B in the blood are not unique to stroke, claiming that the rise occurs in other neurological disorders with similar symptoms [26].
During carotid clamping, seven (10.9%) of the patients who needed shunting developed cerebral ischaemia (neurological deficit). These findings for CEA in conscious patients are similar to those reported by Hans [27] (10%), Evans [28] (9.7%), Calligaro [29] (7.2%), Stroughton [30] (14%), and Rockman [31] (11%). Of the 7 patients who required shunting due to a lack of compensatory blood flow, five patients were in the S100B-positive group. With a sensitivity of 71.4 percent and a specificity of 75.4 percent, we were able to predict neurological alterations in connection to an increase in the S100B marker. A 22.5 percent rise in neurological symptoms was used as the threshold value. Because of the lengthy test process, measuring serum S100B protein for perioperative neuromonitoring is currently not possible. Existing assays lack sensitivity and are difficult to use, and they often take 3 hours to complete. For the measurement of S100B protein, a quick and sensitive immunoassay would be required.
Furthermore, when compared to results acquired after carotid surgery under general anaesthetic, the observed rise in serum S100B protein during transverse clamp in LA was less pronounced (GA). In our study, the average rise in S100B was 12 percent. During CEA in the LA, Aleksic [4] observed a rise of 18% in serum S100B levels. Jaranyi [32] found a 170 percent increase in serum S100B levels in patients undergoing CEA while under GA. This indicates relevant global cerebral ischaemia. S100B levels and cerebral ischaemia alterations in patients undergoing CEA under LA are unlikely to apply to patients undergoing CEA under GA [29].
Symptomatic patients had greater preclamp S100B concentrations than asymptomatic patients. The median baseline serum S100B protein levels were 0.037 g/L and 0.030 g/L, respectively. The difference (P = 456) was not statistically significant. S100B protein peak levels could be identified as early as 20 minutes after cerebral injury. Its biological half-life ranges from 30 to 113 minutes, and it is quickly excreted by the kidneys. Concentrations return to near pre-clamp levels 24 hours after surgery, and by the third post-operative day, they recover completely. In our investigation, all symptomatic patients were operated on more than 72 hours after the onset of carotid symptoms. There were no expected significant variations in baseline S100B protein levels between asymptomatic and symptomatic individuals before surgery. Dragas [10] discovered that symptomatic patients had considerably greater preclamp S100B concentrations. He attributed this to greater embolic potential of symptomatic carotid plaques.
In the group with neurological symptoms, the decline in rSO2 from the preclamp to the cross-clamp period on the ipsilateral side was not significantly greater (P =675). Furthermore, the low AUC value shows that the link between rSO2 percentage reduction and neurological symptoms is at best shaky. In recognizing patients with neurological symptoms, the relative decline in rSO2 is neither sensitive (57.1%) nor specific (63.2%). These findings do not support cerebral oximetry as the sole monitoring modality during carotid endarterectomy. When comparing groups, the absolute lowest value of rSO2 on the contralateral side was statistically different (P =042). This has no clinical implications because baseline rSO2 measurements vary greatly between individuals [33,34]. The range of baseline rSO2 values is large, ranging from 47% to 86%. Rather than using absolute numbers, relative changes in rSO2 should be used [35]. One of the existing rSO2 monitoring’s drawbacks is that the oxygen sensor can only be utilized on hairless scalps.
Other areas of the brain may acquire focal cerebral ischaemia without the drop in rSO2 seen by the scalp sensors [20]. After an abrupt fall in blood flow in brain tissue (which may involve an exponential decrease in tissue oxygenation) with compensation for blood pressure variations, secondary dilatation of the microcirculation due to autoregulation or opening of collateral arteries occurs [36]. Even if intracranial collateral anastomoses exist, they are insufficient to sustain baseline intracerebral oxygen saturation in the brain. The presence of moderate reductions in rSO2 has not been linked to cerebral hypoperfusion severe enough to cause cerebral ischaemia [1]. The crucial rSO2 drop that the brain can endure is unknown.
One of the existing rSO2 monitoring’s drawbacks is that the oxygen sensor can only be utilized on hairless scalps. Other areas of the brain may develop focal cerebral ischaemia without a drop in rSO2 registered by the scalp sensors [20]. After an abrupt fall in blood flow in brain tissue (which may involve an exponential decrease in tissue oxygenation) with compensation for blood pressure variations, secondary dilatation of the microcirculation due to autoregulation or opening of collateral arteries occurs [36]. Even if intracranial collateral anastomoses exist, they are insufficient to sustain baseline intracerebral oxygen saturation in the brain. The presence of moderate reductions in rSO2 has not been linked to cerebral hypoperfusion severe enough to cause cerebral ischaemia [1]. The crucial rSO2 drop that the brain can endure is unknown.
A 13 percent (13.4 percent) reduction in rSO2 was found to be best for detecting individuals with neurological symptoms in our study, with sensitivity and specificity of 57.1 percent and 63.2 percent, respectively. The results were identical to those of Al- Rawi [21] and Mille [22], who used cut-off values of 13% and 12% (11.7%), respectively. There were 8 patients with contralateral carotid occlusion among them. One exhibited neurological symptoms, one had an elevated S100B level in the blood, and two had a considerable drop in rSO2. The need for a shunt is not consistently predicted by contralateral carotid occlusion. Two patients experienced a cerebrovascular insult (CVI). The patient suffering CVI during the procedure exhibited the largest rise in serum S100B protein of all the patients, but her rSO2 was normal. Higher blood S100B protein levels with a late peak are linked to more extensive cerebral injury. In patients with subclinical cerebral tissue death, serum levels are lower and peak increasingly earlier [37]. On the sixth day after the operation, the second patient developed a CVI. Although there was a considerable rise in serum S100B protein, rSO2 remained normal. The CVI attack was predicted by neurological instability and an elevated level of S100B. This was not the case with rSO2.
Limitations
The study had several limitations. First, despite the huge patient group, it was constrained by the small number of patients who experienced neurological symptoms during the surgery. Second, the reported higher S100B levels in the blood of hypertension patients relative to healthy controls made employing the S100B biomarker to diagnose cerebral ischaemia challenging [38]. In terms of serum S100B increase, we did not compare the hypertensive and nonhypertensive categories. Third, different surgeons may assess neurological signs differently during surgery.
Conclusion
Awake neuromonitoring has been found to provide a sensitive and direct evaluation of brain tissue perfusion and is specific to CEA under LA. Although there was a favourable connection between CEA and an increase in serum S100B protein, due to the long assessment time, serum S100B monitoring was not practicable (usually 3 hours). The small number of patients in the study makes it impossible to draw clear conclusions, particularly in the group of people who suffer from neurological problems. Future research will either support or refute our findings.
References
- Williams IM, Mead G, Picton AJ, Farrell A, Mortimer AJ, et al. (1995) The influence of contralateral carotid stenosis and occlusion on cerebral oxygen saturation during carotid artery surgery. Eur J Vasc Endovasc Surg 10(2):198-206.
- Salvian AJ, Taylor DC, Hsiang YN, Hildebrand HD, Litherland HK, et al. (1997) Selective shunting with EEG monitoring is safer than routine shunting for carotid endarterectomy. Cardiovasc Surg 5(5): 481-485.
- Beese U, Langer H, Lang W, Dinkel M (1998) Comparison of nearinfraredspectroscopy and somatosensory evoked potentialsfor the detection of cerebral ischemia during carotidendarterectomy. Stroke 29(10): 2032-2037.
- Aleksic M, Heckenkamp J, Reichert V, Gawenda M, BrunkwallJ (2007) S-100B release during carotid endarterectomy under localanesthesia. Ann Vasc Surg 21(5): 571-575.
- Skitek M, Jerin A (2016) Proenkefalin A and protachykinin inischemic neurological complications after cardiac surgery. Med Glas (Zenica)13(1): 8-13.
- Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, et al. (2006) Serum S-100B protein as abiochemical marker of brain injury: a review of currentconcepts. Curr Med Chem 13(30): 3719-3731.
- Arfvidsson B, Nilsson TK, Norgren L (2015) S100B concentrationsincrease perioperatively in jugular vein blood despitelimited metabolic and inflammatory response to clinicallyuneventful carotid endarterectomy. Clin Chem Lab Med 53(1): 111-117.
- Capoccia L, Sbarigia E, Rizzo A, Mansour W, Speziale F (2012) Silentstroke and cognitive decline in asymptomatic carotid stenosisrevascularization. Vascular 20(4): 181-187.
- Brightwell RE, Sherwood RA, Athanasiou T, Hamady M, Cheshire NJ (2007) The neurological morbidity of carotid revascularisation:using markers of cellular brain injury to compare CEA and CAS. Eur J Vasc Endovasc Surg 34(5): 552-560.
- Dragas M, Koncar I, Opacic D, Ilic N, Maksimovic Z, et al. (2015) Fluctuations of serum neuron specific enolase and protein S-100B concentrations in relation to the use of shunt during carotid endarterectomy. PLoS One 10(4): e0124067.
- Capoccia L, Speziale F, Gazzetti M, Mariani P, Rizzo A, et al. (2010) Comparative study on carotid revascularisation (endarterectomy vs stenting) using markers of cellular brain injury, neuropsychometric tests, and diffusion-weighted magnetic resonance imaging. J Vasc Surg 51(3): 584-591.
- Sahlein DH, Heyer EJ, Rampersad A, Winfree CJ, Solomon RA, et al. (2003) Failure of intraoperative jugular bulb S-100B and neuron-specific enolase sampling to predict cognitive injury after carotid endarterectomy. Neurosurgery 53(6): 1243-1249.
- Connolly ES, Winfree CJ, Rampersad A, Sharma R, Mack WJ, et al. (2001) Serum S100B protein levels are correlated with subclinical neurocognitive declines after carotid endarterectomy. Neurosurgery 49(5): 1076-1082.
- Alserr AH, Elwan H, Antonopoulos CN, Abdelreheem A, Elmahdy H, et al. (2019) Using serum s100-β protein as a biomarker for comparing silent brain injury in carotid endarterectomy and carotid artery stenting. Int Angiol 38(2): 136-142.
- Kuzhuget R, Starodubtsev V, Ignatenko P, Starodubtseva A, Voroshilina O, et al. (2017) The ole of stump pressure and cerebral oximetry in predicting ischaemic brain damage during carotid endarterectomy. Brain Inj 31(13–14): 1944-1950.
- Ge YL, Li X, Gao JU, Zhang X, Fang X, et al. (2016) Beneficial effects of intravenous dexmedetomidine on cognitive function and cerebral injury following a carotid endarterectomy. Exp Ther Med 11(3): 1128-1134.
- Nagy B, Woth G, Mérei Á, Nagy L, Lantos J, et al. (2016) Perioperative time course of matrix metalloproteinase-9(MMP-9), its tissue inhibitor TIMP-1 & S100B protein in carotid surgery. Indian J Med Res 143(2): 220-226.
- Falkensammer J, Oldenburg WA, Hendrzak AJ, Neuhauser B, Pedraza O, et al. (2008) Evaluation of subclinical cerebral injury and neuropsychologic function in patients undergoing carotid endarterectomy. Ann Vasc Surg 22(4): 497-504.
- Manwaring ML, Durham CA, McNally MM, Agle SC, Parker FM, et al. (2010) Correlation of cerebral oximetry with internal carotid artery stump pressures in carotid endarterectomy. Vasc Endovascular Surg 44(4): 252-256.
- Samra SK, Dorje P, Zelenock GB, Stanley JC (1996) Cerebral oximetry in patients undergoing carotid endarterectomy under regional anesthesia. Stroke 27(1): 49-55.
- Al-Rawi PG, Kirkpatrick PJ (2006) Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke 37(11): 2720-2725.
- Mille T, Tachimiri ME, Klersy C, Ticozzelli G, Bellinzona G, et al. (2004) Near infrared spectroscopy monitoring during carotid endarterectomy: which threshold value is critical? Eur J Vasc Endovasc Surg 27(6): 646-650.
- Kapural M, Krizanac-Bengez LJ, Barnett G, Perl J, Masaryk T, et al. (2002) Serum S-100β as a possible marker of bloodbrain barrier disruption. Brain Res 940(1-2): 102-104.
- González-García S, González-Quevedo A, Peña-Sánchez M, Menéndez-Saínz C, Fernández-Carriera R, et al. (2012) Serum neuron-specific enolase and S100 calcium binding protein B biomarker levels do not improve diagnosis of acute stroke. J R Coll Physicians Edinb 42(3): 199-204.
- Jickling GC, Sharp FR (2011) Blood biomarkers of ischaemic stroke. Neurotherapeutics 8(3): 349-360.
- Dassan P, Keir G, Brown MM (2009) Criteria for a clinically informative serum biomarker in acute ischaemic stroke: a review of S100B. Cerebrovasc Dis 27(3): 295-302.
- Hans SS, Jareunpoon O (2007) Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg 45(3): 511-515.
- Evans WE, Hayes JP, Waltke EA, Vermilion BD (1985) Optimal cerebral monitoring during carotid endarterectomy; neurologic response under local anesthesia. J Vasc Surg 2(6): 775-777.
- Calligaro KD, Dougherty MJ (2005) Correlation of carotid artery stump pressure and neurological changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg 42(4): 684-689.
- Stroughton J, Nath RL, Abbott WM (1998) Comparison of simultaneous electroencephalographic and mental status monitoring during carotid endarterectomy with regional anesthesia. J Vasc Surg 28(6): 1014-1023.
- Rockman CB, Riles TS, Gold M, Lamparello PJ, Giangola G, et al. (1996) A comparison of regional and general anesthesia in patients undergoing carotid endarterectomy. J Vasc Surg 24(6): 946-956.
- Jaranyi Z, Szekely M, Bobek I, Galfy I, Geller L, et al. (2003) Impairment of blood-brain barrier integrity during carotid surgery as assessed by serum S-100B protein concentrations. Clin Chem Lab Med 41(10): 1320-1322.
- Duffy CM, Manninen PH, Chan A, Kearns CF (1997) Comparison of cerebral oximeter and evoked potential monitoring in carotid endarterectomy. Can J Anaesth 44(10): 1077-1081.
- Hernandez-Avila G, Dujovny M, Slavin KV, Luer MS, Nijensohn E, et al. (1995) Use of transcranial cerebral oximetry to monitor regional cerebral oxygen saturation during neuroendovascular procedures. AJNR Am J Neuroradiol 16(8): 1618-1625.
- Duncan LA, Ruckley CV, Wildsmith JA (1995) Cerebral oximetry: a useful monitor during carotid artery surgery. Anaesthesia 50(12): 1041-1045.
- Lam JM, Smielewski P, al-Rawi P, Griffiths P, Pickard JD, et al. (1997) Internal and external carotid contributions to near-infrared spectroscopy during carotid endarterectomy. Stroke 28(5): 906-911.
- Jonsson H, Johnsson P, Alling C, Backstrom M, Bergh C, et al. (1999) S100beta after coronary artery surgery:release pattern, source of contamination, and relation to neuropsychometric outcome. Ann Thorac Surg 68: 2202-2208.
- González-Quevedo A, González García S, Fernández Concepción O, Rosaralis Santiesteban Freixas, Luis Quevedo Sotolongo, et al. (2011) Increased serum S-100B and neuron specific enolase potential markers of early nervous system involvement in essential hypertension. Clin Biochem 44(2-3): 154-159.

 Research Article
Research Article