Abstract
Introduction: The optimal use of advanced therapy, including systemic or catheter-directed thrombolysis, for patients hospitalized with pulmonary embolism (PE) is uncertain.
Methods: Retrospective cohort study using multivariable regression analysis comparing outcomes of 30-day and 7-day mortality, length of stay, cost, major bleeding, non-critical bleeding, and any bleeding in patients with acute PE treated with advanced therapy vs. anticoagulation alone.
Results: A total of 607 patients met inclusion criteria, 44 patients (7.2%) received advanced therapy, predominantly IVC filter placement (n=35), while the remainder received anticoagulation alone. No significant difference in 30-day mortality was observed between treatment with advanced therapy or anticoagulation alone (HR [95% CI] =0.96 [0.44-2.09]). Secondary outcomes of length of stay and normalized mean cost were increased among patients treated with advanced therapy (length of stay [95% CI]; 8.37 days [4.48-12.26] vs. 3.84 days [3.34-4.33], p=0.002; normalized mean cost [95% CI]; 3.03 [1.62-4.43] vs. 1.00 [0.87-1.13], p<0.001). Major bleeding was significantly increased among patients receiving advanced therapy (HR=9.05 [2.21-9.98], p <0.001).
Conclusions: Advanced therapy for acute pulmonary embolism is associated with increased hospital length of stay, total cost, and major bleeding with no significant difference in 30-day mortality. Further research is needed to identify the most effective and highest value opportunities for advanced therapies in acute pulmonary embolism management.
Keywords: Arthroscopic Soft Tissue Stabilization; Primary Procedures; Shoulder Instability
Abbreviations: PE: Pulmonary Embolism; CDT: Catheter-Directed Thrombolysis; ECMO: Extracorporeal Membrane Oxygenation; CTPA: Computed Tomography of the Pulmonary Arteries; NLP: Natural Language Processing; ICD: International Classification of Diseases; IVC: Inferior Vena Cava; ACCP: American College of Chest Physicians; LOS: Length of Stay; ISTH: International Society on Thrombosis and Haemostasis; SD: Standard Deviation; IQR: Interquartile Range; HR: Hazard Ratio; CCI: Charlson Comorbidity Index
Introduction
Venous thromboembolism is a common cause of hospitalization with a high mortality rate [1,2]. Current guidelines recommend anticoagulation alone for patients that are hemodynamically stable and considered to have a low-risk Pulmonary Embolism (PE) [3,4]. Thrombolytic therapy is recommended for patients with hypotension, considered a high-risk PE, in the absence of contraindications [3-5]. The optimal management of intermediaterisk PE is less clear. Some have proposed treating intermediaterisk PE with intravenous thrombolytics; however, trials have demonstrated increased rates of bleeding without mortality benefits [6-9]. Other advanced therapies have been developed to treat life-threatening PE, including Catheter-Directed Thrombolysis (CDT), suction thromboembolectomy, surgical embolectomy and Extracorporeal Membrane Oxygenation (ECMO), [10-13] but robust evidence supporting their use is lacking.
Because the optimal management of intermediate- and high-risk PE with advanced therapies remains uncertain, some institutions have developed a multidisciplinary team of specialists to determine the best therapeutic approach for patients presenting with an acute PE called a PE response team (PERT) [14]. In spite of multiple institutions deploying PERTs, evidence on the effectiveness of a PERT is limited. In some reports, PERTs have led to increased utilization of advanced therapies, particularly catheter-directed therapy, without clearly demonstrating improvement in outcomes such as mortality, bleeding rates or length of stay [15,16]. Our institution is an academic medical center with ready access to advanced therapies for PE that does not rely on a PERT to guide treatment decisions. We believe our center is well-positioned to evaluate whether advanced therapies are associated with improved outcomes compared to anticoagulation alone in absence of a PERT. We hypothesized that patients who received advanced therapies compared to anticoagulation alone would have increased rates of bleeding, higher total direct costs and longer length of stay, without a difference in short-term mortality.
Methods
Study Design
We conducted a retrospective observational cohort study at the University of Utah Medical Center, a 528-bed academic medical center with one of the largest geographic referral areas in the United States. An electronic database query was used to identify patients who underwent chest Computed Tomography of the Pulmonary Arteries (CTPA) and/or ventilation/perfusion (V/Q) scan between April 1, 2017 and April 1, 2019. An internally validated Natural Language Processing (NLP) tool designed to scan and interpret the CTPA, or V/Q radiology report was used to identify PE events. This NLP tool has a sensitivity of 96.0% and a specificity of 97.7% for identification of acute PE. We used the NLP tool to identify eligible patients based on previous reports suggesting International Classification of Diseases (ICD) discharge codes cannot reliably identify acute thromboembolic events [17,18]. Baseline patient demographics, vital signs, medical comorbidities, lab results, medications, interventions, admission date, discharge date, and bleeding events with dates were extracted from the electronic health record. Four study investigators performed manual chart reviews to validate study data regarding presence of acute PE, echocardiography, thrombolytic eligibility, advanced therapy for PE, and bleeding events. Each chart was independently reviewed by two study investigators, with any discrepancies adjudicated by a third investigator (SAJ) blinded to the identity of the initial reviewers.
Patient Population
Inclusion criteria were age greater than 18 years and the presence of a PE identified by the NLP tool during the study period. Exclusion criteria included chronic PE or absence of PE identified through manual chart review. Study subjects were assigned to one of two cohorts based on the treatment they received for their acute PE:
1) Anticoagulation alone; or
2) Advanced therapy.
Advanced therapy was defined as receipt of systemic thrombolysis, CDT, Inferior Vena Cava (IVC) filter placement, ECMO, and/or surgical embolectomy. Thrombolytic ineligibility was defined as the presence of any of the following: active bleeding or DIC; cerebral vascular lesion or intracranial neoplasm; ischemic stroke in previous three months; prior intracranial hemorrhage; major surgery, trauma, obstetric delivery, GI bleeding, or invasive procedure within the previous 14 days intracranial, spinal, or retinal surgery within previous 30 days; CPR >10 minutes. These are based on our institutional criteria, adapted from the American College of Chest Physicians (ACCP) guidelines [3]. Patients were further stratified by pulmonary embolism severity index (PESI) [19] and PE location (main, interlobar, lobar, segmental, or subsegmental pulmonary arteries; and bilateral or unilateral involvement).
Study Outcomes
The primary efficacy outcome was 30-day mortality after acute PE identification. Secondary efficacy outcomes included 7-day mortality, hospital length of stay (LOS), and normalized mean of the total direct cost. The primary safety outcome was major bleeding within 90 days of PE diagnosis. Major bleeding was defined as fatal bleeding or bleeding into a critical site according to the International Society on Thrombosis and Haemostasis (ISTH) [20]. ICD codes were used to identify patients with bleeding into a critical location. We did not apply the 2gm/dL decrease in hemoglobin criteria proposed in the ISTH definition as we believed this criterion was subject to false positive results from reasons other than bleeding (eg. haemolysis, haemodilution, phlebotomy, anaemia of acute illness). Secondary safety outcomes included any bleeding, non-critical bleeding, and procedure-related bleeding events. Non-critical bleeding was defined as non-fatal bleeding into non-critical locations. Procedure-related bleeding was defined as bleeding associated with a recent surgical or interventional procedure. ICD codes use to identify bleeding events are listed in Appendix Table 1. All bleeding events and dates identified by ICD codes were confirmed on manual chart review. Total direct costs were determined by our institutionally derived Value Driven Outcomes tool [21].
Statistical Analysis
Baseline characteristics and unadjusted outcomes are reported as frequency and percent, mean with standard deviation (SD) for normally distributed variables, and median with interquartile range (IQR) for non-normally distributed variables. Baseline characteristics and unadjusted outcomes were compared using the Chi-squared or Fisher’s exact test, Student’s t-test, or Wilcoxon rank sum where appropriate. Multivariable regression analysis using generalized linear models with a log-link function and gamma distribution was used for continuous outcomes (eg. length of stay, cost). Survival analysis using univariate and multivariable Cox regression was used to compare the primary and secondary efficacy and safety outcomes, reported as a hazard ratio (HR) with 95% confidence interval (95% CI). Kaplan-Meier curves were used to graphically display differences in mortality and bleeding events. Multivariable logistic regression was used for binary outcomes. Covariates included in regression models were Charlson Comorbidity Index (CCI) [22], thrombolytic eligibility [4], and PESI score [19]. We chose CCI to adjust for any potential differences in chronic comorbidities, thrombolytic eligibility to adjust for differences in baseline bleeding risk, and PESI score as an indicator of acute illness related to the PE. Adjusted continuous outcomes were estimated using marginal effects at the means [23]. Total direct costs were normalized using the mean total direct cost of admission for patients receiving anticoagulation alone as the normalizing value. A p-value cutoff of 0.05 was used to determine statistical significance. Stata/IC version 16.1 (StataCorp, College Station, TX) was used for all analyses.
Results
Patient Characteristics
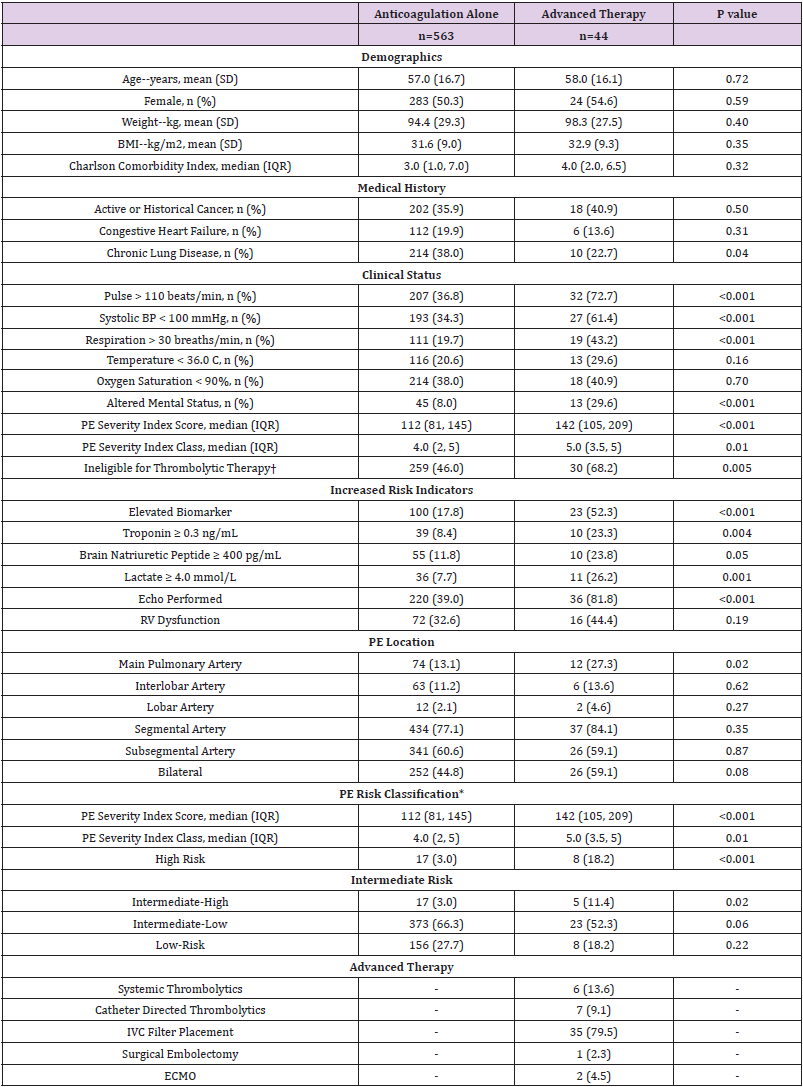
A total of 741 patients with either a CTPA or V/Q scan performed during the study period were identified. Of these, 134 patients met exclusion criteria, leaving 607 patients with an acute PE for analysis (Appendix Figure 1). 563 patients received anticoagulation alone and 44 received advanced therapy for PE treatment. Most patients in the advanced therapy group (79.5%) received an IVC filter, 13.6% received systemic thrombolysis and 9.1% received catheter-directed thrombolysis. Baseline patient demographics did not differ significantly between groups (Table 1). Overall, patients in the advanced therapy cohort presented with greater vital sign derangements and higher PESI scores compared to the anticoagulation alone cohort (median PESI score [IQR]; 112 [81, 145] vs. 142 [105, 209], p <0.001). More patients in the advanced therapy cohort were considered ineligible for thrombolytic therapy (46.0% vs. 68.2%, p <0.001). Elevated biomarkers suggestive of right ventricular (RV) dysfunction (eg. troponin, brain-natriuretic peptide, lactate) were observed more frequently among advanced therapy patients (Table 1), although echocardiographic evidence of RV dysfunction was not significantly different (p =0.19) between the advanced therapy (44.4%) and anticoagulation alone (32.6%) cohorts. More patients in the advanced therapy cohort had a PE identified in the main pulmonary artery compared to the anticoagulation alone cohort (27.3% vs. 13.1%, p = 0.02).
Table 1: Baseline Patient Characteristics of Patients Treated with Anticoagulation-Alone versus Advanced Therapy for Acute Pulmonary Embolism.
Note: †Defined as presence of active bleeding or DIC; cerebral vascular lesion or intracranial neoplasm; ischemic stroke in previous three months; prior intracranial hemorrhage; major surgery, trauma, obstetric delivery, GI bleeding, or invasive procedure within the previous 14 days intracranial, spinal, or retinal surgery within previous 30 days; CPR >10 minutes. *Risk classification based on European Society of Cardiology guidelines for the diagnosis and management of acute pulmonary embolism. Abbreviations: BMI= body mass index; BP= blood pressure; PE= pulmonary embolism; RV= right ventricle; IVC= inferior vena cava; ECMO= extracorporeal membrane oxygenation
Outcomes
Primary and Secondary Efficacy Outcomes
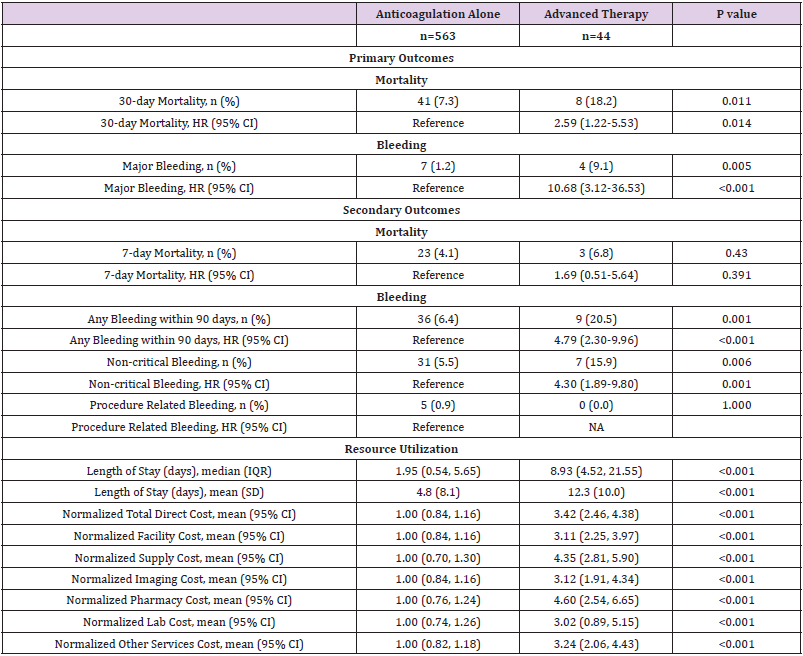
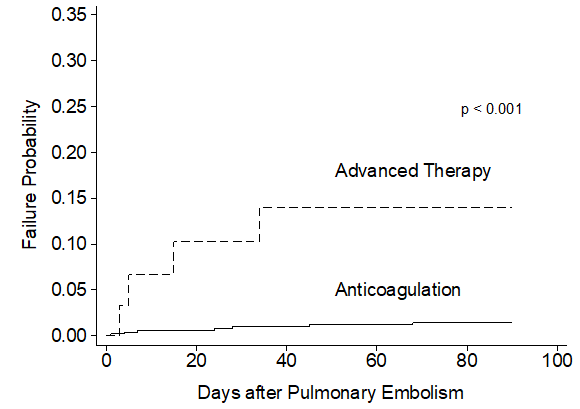
In unadjusted comparisons, the primary efficacy outcome of 30-day mortality was observed more often in patients treated with advanced therapy (18.2%) compared to patients treated with anticoagulation-alone (7.3%) (hazard ratio [95% CI]; HR=2.59 [1.22-5.53], p =0.014) (Table 2, Figure 1). No significant differences were noted in the secondary outcome of 7-day mortality (HR= 1.69 [0.51-5.64], p =0.391. The median LOS was nearly 7 days longer for patients treated with an advanced therapy (median LOS [IQR]; 8.93 [4.52, 21.55] days vs. 1.95 [0.54, 5.65] days, p <0.001). Normalized mean total direct costs were more than 3-fold higher for patients treated with an advanced therapy vs. anticoagulation alone (normalized mean cost [95% CI], 3.42 [2.46-4.38] vs. 1.00 [0.84-1.16], p <0.001).
Table 2: Unadjusted Primary and Secondary Outcomes of Anticoagulation-Alone versus Advanced Therapy for Acute Pulmonary Embolism.
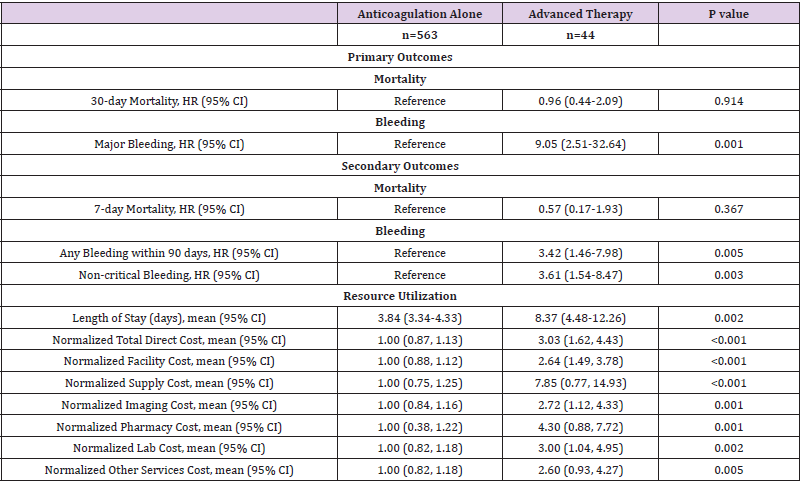
Following adjustments for PESI score, CCI, and thrombolytic eligibility, the observed differences in 30-day mortality were no longer apparent between the advanced therapy and anticoagulationalone cohorts (HR=0.96 [0.44-2.09], p =0.914) (Table 3). No significant differences were observed in 7-day mortality (HR=0.57 [0.17-1.93], p =0.367). The adjusted mean LOS was 4.53 days longer for patients treated with advanced therapy relative to those treated with anticoagulation alone (mean LOS [95% CI]; 8.37 days [4.48-12.26] vs. 3.84 days [3.34-4.33], p =0.002). Similarly, adjusted normalized mean costs remained more than 3-fold higher among patients treated with advanced therapy (normalized mean cost [95% CI]; 3.03 [1.62-4.43] vs. 1.00 [0.87-1.13], p <0.001).
Table 3: Adjusted Primary and Secondary Outcomes of Anticoagulation-Alone versus Advanced Therapy for Acute Pulmonary Embolism.
Note: Results adjusted for Charlson comorbidity index, thrombolytic ineligibility, and Pulmonary Embolism Severity Index score.
Primary and Secondary Safety Outcomes
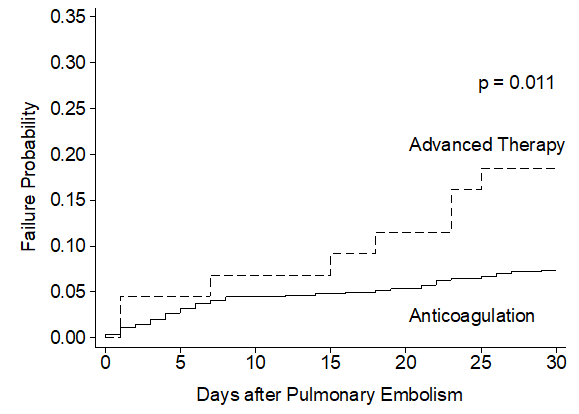
Unadjusted analysis demonstrated the primary safety outcome of major bleeding was observed more frequently among patients treated with advanced therapy (9.1%) compared to patients receiving anticoagulation alone (1.2%) (HR=10.68 [3.12-36.53], p <0.001) (Table 2). Most major bleeding events occurred within the first 20 days following advanced therapy as demonstrated in Figure 2. The secondary outcomes of any bleeding event (HR= 4.79 [2.30- 9.96], p <0.001) and non-critical bleeding (HR= 4.30 [1.89-9.80], p =0.001) were also significantly increased and occurred early in the advanced therapy cohort (Appendix Figure 2). Following multivariable adjustment, major bleeding remained significantly increased among patients receiving advanced therapy (HR=9.05 [2.21-9.98], p <0.001). The secondary outcomes of any bleeding (HR= 3.42 [1.46-7.98], p =0.005) and non-critical bleeding (HR= 3.61 [1.54-8.47], p =0.003) remained significantly increased in the advanced therapy cohort after adjustments.
Figure 2: Crude Incidence Rate of 90-Day Major Bleeding Events following Pulmonary Embolism Diagnosis.
Discussion
In this retrospective cohort study at a single academic medical center, we examined the relationship between advanced therapy for acute PE and patient outcomes. Our multivariable analysis identified a significant association between utilization of advanced therapy and increases in hospital LOS, total cost, and bleeding likelihood with no significant difference in 30-day mortality, compared to treatment with anticoagulation alone. Optimal patient selection for use of advanced therapies in acute PE remains uncertain, particularly in the case of intermediate-risk PE. One solution to address this uncertainty has been the formation of a multidisciplinary PERT. In our study at an academic medical center without a PERT, 7.2% of patients received advanced therapy, the majority of these being an IVC filter, with 1.2% receiving CDT. Adoption of PERTs at these institutions was associated with increased utilization of advanced therapies at other academic institutions without PERTs or prior to PERTs, where IVC filters are generally utilized in < 10% of patients and CDT < 2%. [15,16,24] Adoption of PERTs at these institutions was associated with increased utilization of advanced therapies, with a 10-fold increase in CDT utilization. Across the PERT consortium utilization of advanced therapy ranges from 16-46%, [15,16,25]. However, the benefit of increased utilization of advanced therapies remains uncertain without consistently improved clinical outcomes, [15,16,25-29].
We observed the 30-day mortality was significantly higher in the patients who received advanced therapy (18.2% vs 7.3%), however, after adjusting for differences in PE severity and patient characteristics, no mortality difference was observed (HR=0.96 [0.44-2.09], p =0.914). This may be due to a lack of mortality benefit attributable to advanced therapies in these patients or limited power to detect a relatively uncommon outcome. We did identify a significantly increased risk of major bleeding within 90 days (HR=9.05 [2.21-9.98], p <0.001) in patients treated with advanced therapies. Because the overwhelming majority of advanced therapies utilized in our center were IVC filters, which are often placed due to elevated bleeding risk, this observation may be due to residual confounding by indication. We also compared LOS and total costs of care, which we found to be significantly higher in the advanced therapy group. This may be partially explained by the cost of the advanced therapy itself, however, increased costs in all categories (facilities, supply, imaging, pharmacy, lab) suggest total cost differences are not entirely explained by treatment modality for pulmonary embolism. It is conceivable that patients identified with a high- or intermediate-risk PE necessitating advanced therapy may have underlying comorbidities (eg. cancer) or acute illness that requires more resources than patients who are well enough to receive anticoagulation therapy alone.
Strengths of this study include thorough review of each clinical chart by multiple study investigators (who are all practicing hospitalists) to manually validate the diagnosis of acute pulmonary embolism, echocardiographic findings, and any clinical contraindications to thrombolytic therapy. Our normalized cost analysis and evaluation of categorized cost is novel in this field and of importance due to increasing consideration of high value care in health systems. Our study is not without limitations. We cannot exclude the possibility of unmeasured or residual confounding, though we did control for well-established prediction scores for pulmonary embolism severity, comorbidities, and bleeding risk in our multivariable model. Our analysis was conducted within a single academic medical canter, and our findings may not be generalizable to other populations. Lastly, utilization of advanced therapies was relatively low across our study population, and we lacked sufficient power for further subgroup analysis dependent on type of advanced therapy used.
In conclusion, we observed an association between advanced therapies for acute PE and increased cost of care, LOS, and major bleeding events, without a reduction in mortality. Given previously described significant increases in utilization of advanced therapies after adoption of PERTs, it will be important to carefully monitor patient-centered clinical outcomes with adoption of new care systems. Adequately powered randomized clinical trials are needed to identify the safest and most effective advanced therapies for acute PE management.
References
- (2012) Venous thromboembolism in adult hospitalizations - United States, 2007-2009. MMWR Morb Mortal Wkly Rep 61(22): 401-404.
- Goldhaber SZ, Visani L, De Rosa M (1999) Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 353(9162): 1386-1389.
- Kearon C, Akl EA, Ornelas J, Joseph O, Allen B, et al. (2016) Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest 149(2): 315-352.
- Konstantinides SV, Meyer G, Becattini C, Hector B, GJ Geersing, et al. (2020) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41(4): 543-603.
- Jaff MR, McMurtry MS, Archer SL, Mary C, Neil G, et al. (2011) Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 123(16): 1788-1830.
- Meyer G, Vicaut E, Danays T, G Abnelli, Cecilia B, et al. (2014) Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med 370(15): 1402-1411.
- Konstantinides SV, Vicaut E, Danays T, L Bertoletti, Jan BW, et al. (2017) Impact of Thrombolytic Therapy on the Long-Term Outcome of Intermediate-Risk Pulmonary Embolism. J Am Coll Cardiol 69(12): 1536-1544.
- Chatterjee S, Chakraborty A, Weinberg I, Mitul K, Robert LW, et al. (2014) Thrombolysis for pulmonary embolism and risk of all-cause mortality, major bleeding, and intracranial hemorrhage: a meta-analysis. Jama 311(23): 2414-2421.
- Marti C, John G, Konstantinides S, C Combescure, Olivier S, et al. (2015) Systemic thrombolytic therapy for acute pulmonary embolism: a systematic review and meta-analysis. Eur Heart J 36(10): 605-614.
- Kucher N, Boekstegers P, Muller OJ, C Kupatt, Thomas H, et al. (2014) Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation 129(4): 479-486.
- Piazza G, Hohlfelder B, Jaff MR, Kenneth O, Tod CE, et al. (2015) A Prospective, Single-Arm, Multicenter Trial of Ultrasound-Facilitated, Catheter-Directed, Low-Dose Fibrinolysis for Acute Massive and Submassive Pulmonary Embolism: The SEATTLE II Study. JACC Cardiovasc Interv 8(10): 1382-1392.
- Lee T, Itagaki S, Chiang YP, Egorova NN, Adams DH, et al. (2018) Survival and recurrence after acute pulmonary embolism treated with pulmonary embolectomy or thrombolysis in New York State, 1999 to 2013. J Thorac Cardiovasc Surg 155(3): 1084-1090.
- Pasrija C, Kronfli A, Rouse M, Maxwell RBS, Gregory J, et al. (2018) Outcomes after surgical pulmonary embolectomy for acute submassive and massive pulmonary embolism: A single-center experience. J Thorac Cardiovasc Surg 155(3): 1095-1106.
- Kabrhel C, Jaff MR, Channick RN, Baker JN, Rosenfield K (2013) A multidisciplinary pulmonary embolism response team Chest 144(5): 1738-1739.
- Rosovsky R, Chang Y, Rosenfield K, Richard C, Michael RJ, et al. (2019) Changes in treatment and outcomes after creation of a pulmonary embolism response team (PERT), a 10-year analysis. J Thromb Thrombolysis 47(1): 31-40.
- Xenos ES, Davis GA, He Q, Green A, Smyth SS (2019) The implementation of a pulmonary embolism response team in the management of intermediate- or high-risk pulmonary embolism. J Vasc Surg Venous Lymphat Disord 7(4): 493-500.
- Lawrence K, Joos C, Jones AE, Johnson SA, Witt DM (2019) Assessing the accuracy of ICD-10 codes for identifying acute thromboembolic events among patients receiving anticoagulation therapy. J Thromb Thrombolysis 48(2): 181-186.
- Fang MC, Fan D, Sung SH, Daniel MW, John RS, et al. (2017) Validity of Using Inpatient and Outpatient Administrative Codes to Identify Acute Venous Thromboembolism: The CVRN VTE Study. Med Care 55(12): e137-e143.
- Aujesky D, Obrosky DS, Stone RA, Thomas EA, Arnaud P, et al. (2005) Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 172(8): 1041-1046.
- Schulman S, Kearon C (2005) Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3(4): 692-694.
- Kawamoto K, Martin CJ, Williams K, Ming CT, Charlton GP, et al. (2015) Value Driven Outcomes (VDO): a pragmatic, modular, and extensible software framework for understanding and improving health care costs and outcomes. J Am Med Inform Assoc 22(1): 223-235.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5): 373-383.
- Williams R (2012) Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata Journal 12(2): 308-331.
- Freeland ZK, Clayton JT, Rosenblatt RL (2019) Management of pulmonary embolism at a large academic hospital. Proc (Bayl Univ Med Cent) 32(1): 9-13.
- Schultz J, Giordano N, Zheng H, Blair AP, Geoffy DB, et al. (2019) EXPRESS: A Multidisciplinary Pulmonary Embolism Response Team (PERT) - Experience from a national multicenter consortium. Pulm Circ 9(3): 2045894018824563.
- Chaudhury P, Gadre SK, Schneider E, Rahul DR, Marcelo G, et al. (2019) Impact of Multidisciplinary Pulmonary Embolism Response Team Availability on Management and Outcomes. Am J Cardiol 124(9): 1465-1469.
- Jen WY, Kristanto W, Teo L Jason P, Hwee SY, et al. (2020) Assessing the Impact of a Pulmonary Embolism Response Team and Treatment Protocol on Patients Presenting with Acute Pulmonary Embolism. Heart Lung Circ 29(3): 345-353.
- Khaing P, Paruchuri A, Eisenbrey JR, Geno JM, Carin FN, et al. (2020) First year experience of a pulmonary embolism response team with comparisons of outcomes between catheter directed therapy versus standard anticoagulation. Hosp Pract (1995) 48(1): 23-28.
- Wright C, Elbadawi A, Chen YL, Dhwani P, Justin M, et al. (2019) The impact of a pulmonary embolism response team on the efficiency of patient care in the emergency department. J Thromb Thrombolysis 48(2): 331-335.

 Research Article
Research Article