ABSTRACT
The application of various drains has been a part of the surgical activity since ancient times. Today’s modern surgery chooses drainage itself and drainage systems according to clear criteria and purpose. The most used sizes of drains were found to be 28F, 32F and 36F in adults and 16F, 20F and 24F in children. The most important criteria for drain selection are drainage efficiency (performance), biostability and biocompatibility. Drainage systems are a common part of postoperative surgical management and are used to remove fluid collection from the abdominal cavity. When draining the peritoneal cavity, it is necessary to be aware of the most common places of fluid accumulation. Abdominal drainage is not easy, especially due to more complicated anatomical conditions and the presence of consciousness and intestinal loops. The aim of this review was to evaluate the evidence supporting the systematic use of abdominal drainage.
Keywords: Drains; Drainage; Abdominal Surgery; Intra-Abdominal Drains
Introduction
Since ancient times, surgical procedures have been associated with conditions, that have either the cause, or effect of evacuating liquid media, that are detrimental to the body. To achieve this effect, various devices, which fall under the collective name “drains”, have been gradually designed, described, and used. We call the drain a simple or more com-plicated aid or a whole system used to evacuate unwanted fluids from the body [1]. The application of various drains has been a part of the surgical activity since the days of Hippocrates, when various metal, bone, gauze or wick preparations and gauze combinations were used as means of passive drainage. The oldest record of the usage of drainage comes from Hippocrates himself (480-377 BC). He used a wooden tube to drain the empyema [2]. Today’s modern surgery is more advanced and precise - it chooses drainage itself and drainage systems according to clear criteria and purpose:
• They remove pathological liquid products (purulent fluids, intestinal contents, effusion, etc.)
• They remove loose intra-abdominal fluids (bile, pancreatic juices, lavage fluids) before they can cause complications.
• They have a restituting effect [1].
Characteristics of Drainage Systems and their Effectiveness
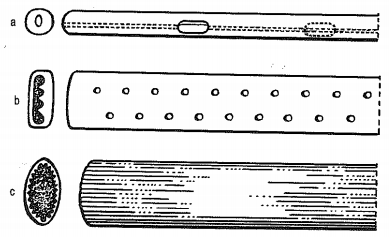
The shape of the drains can be straight or shaped. Curved drains are used in thoracic surgery, where they are applied after surgery on the diaphragm. The end of the drain that remains outside the body can be either funnel-shaped to connect to the connector (inserted from the outside) or beveled for easier passage through the chest wall (inserted during internal surgery) [3]. Drains are manufactured in various lengths and widths. The width of the drain can be marked either by a number according to the Charrier scale, or the so-called French units. On the Charrier scale, the number 1 is equal to 0.3 mm and each subsequent number is 0.3 mm larger (Figure 1) [2]. The most used sizes were found to be 28F, 32F and 36F in adults and 16F, 20F and 24F in children. Polyethylene catheters (Intracarth), “J” -shaped catheters are intended for neonatal age [4]. When choosing the size of the lumen of the drain and the various couplings, it is necessary to proceed from the laws applicable to the dynamics of gases and fluids. Poiseuille’s equation is assumed for the gas flow under the assumption of laminar flow. The air flow is directly proportional to the square of the drain radius. For humid air, which has a turbulent flow, the Fanning equation applies, and so the flow rate depends on the radius of the drain up to the fifth power of the radius [5].
The Most Important Criteria for Drain Selection
Drainage efficiency (performance), biostability and biocompatibility are the most important criteria. Rubber hoses are nowadays rejected due to poor biostability and surface structure deficit. The only exception is their use in T-drainage. As a result of secondary structural changes in the drainage material, which are caused enzymatically, there is a progressive rigidity of the material with increasing storage time in the body. Prolonged intra-abdominal drainage with rubber drains can lead to intestinal erosions. PVC materials should be excluded due to insufficient biocompatibility [6].
Drainage Efficiency
The total flow of the drainage system is the sum of all the individual streams that flow through the openings in the wall of the collecting channel. The flow in the collecting channel is turbulent, and beliefs are formed. An important parameter is the relationship of the sum of the areas of all side holes of the drain (f) to the crosssectional area of the collecting channel (b). Mainly drainage with a large f:b ratio did not initially show any pressure drop. The side openings in the rear part of the collecting channel suck the most, proportionally more than the openings in the front part. In the experiment, the authors tested and evaluated 14 different drainage systems and the following conclusions were drawn:
1. The larger the diameter of the drain, the larger the volume flow. The volume flow is the same in each cross section of the tube.
2. Drainages with a square cross-section at the beginning of the stream show approximately the same volume flow when compared to drains of circular cross-section with the same cross-sectional area size.
3. For suction drainage, a certain wall resistance against collapse must be ensured.
4. With the same geometry, the larger the volume flow, the more side holes of the same size are located on the drain.
5. As the ratio of the sum of the areas of the side openings to the cross-section of the collecting channel increases, the drainage capacity increases [7].
Principles for Abdominal Drainage
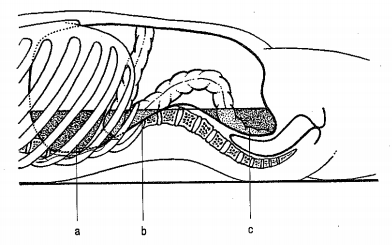
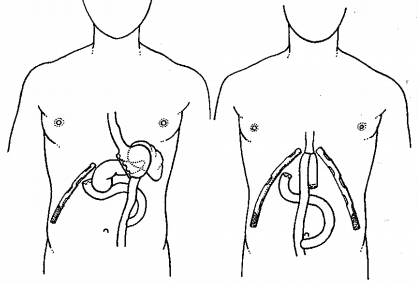
Figure 2: Premises in which fluid accumulates in a lying patient –
a) Subphrenic space on the left,
b) Paracolic space on the left,
c) Douglas space.
The issue of abdominal drainage has undergone great development. The advantages and disadvantages of drainage, questions of when, how, and what to drain during operations are considered. However, there is some consensus. In elective or minor uncomplicated abdominal surgeries (appendectomy, cholecystectomy), most authors are inclined to recommend not draining the abdominal cavity. Drainage is always recommended for potentially complicated surgical procedures where complications may be expected. Drainage should never be conducted by the surgical wound - due to the weakening of the wound and the possibility of postoperative herniation at the site of the drain and the risk of possible infection of the surgical wound. In places where we do not see, the drain is placed by hand. Direct drainage of the drain with the anastomosis should be avoided [8]. The abdominal cavity is an enclosed space (abdominal compartment) bounded by a rigid posterior wall and anterior-moving muscular wall. Muscles form the abdominal cavity with their tension and with their activity participate in locomotion, participate in ventilation, etc. When draining the peritoneal cavity, it is necessary to be aware of the most common places of fluid accumulation (Figure 2). In the vertical position, the lowest stored area of the abdominal cavity is the Douglas space, in the horizontal position the subphrenic space on both sides and also the Douglas space [9].
In the vertical position, the lowest stored area of the abdominal cavity is the Douglas space, and in the horizontal position the subphrenic space on both sides and also the Douglas space. The circulation of fluid between these three spaces is given by the intraabdominal pressure and the gravity of the fluid (Figure 3). The activity of the abdominal muscles is reflected in the change in intraabdominal pressure. In addition to muscle contraction, the increase in intra-abdominal pressure may be caused by acute visceral distension [9,10]. The resting intra-abdominal pressure in the horizontal position reaches values in the range of 0.78-1.5 kPa (8- 15.3 cm H2O). At the stand, the pressure increases due to gravity up to 2.94 kPa at the bottom of the Douglas space. During respiration, the intra-abdominal pressure changes by about 0.4 kPa, but with a severe cough, it can reach values of up to 14.7 kPa (150 cm H2O) [10]. The pressure changes resulting from respiratory movements are greater in the right hypochondria due to the position of the liver than in the left. Data on intra-abdominal pressure in the early postoperative period after laparotomy are interesting. While the intra-abdominal pressure is around 2 kPa on the first postoperative day, the fourth postoperative day can reach up to 6 kPa (61.2 cm H2O). Changes in intra-abdominal pressure also occur during artificial lung ventilation and are reflected in a higher incidence of laparoscopic wound dehiscence. Based on a number of experimental and clinical studies in the 1990s, intra-abdominal hypertension has been shown to cause abdominal compartment syndrome, which is often observed in clinical patients and in patients after severe abdominal trauma [11,12]. An increase in the content of the abdominal cavity as well as the retroperitoneum contributes to the increase of intra-abdominal pressure. Intra-abdominal pressure is transmitted to adjacent areas and has adverse effects on cardiac output, pulmonary ventilation, renal function, and cerebrospinal pressure. Increasing intra-abdominal pressure has an adverse effect on visceral perfusion. The more the intra-abdominal pressure increases and the longer this increase lasts, the more the blood flow through the splanchnic area decreases, which is reflected in the lowering of the pH of the intestinal mucosa [13].
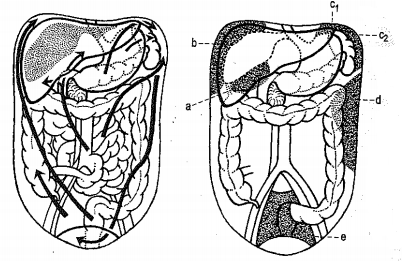
Figure 3: Directions of possible fluid propagation in the abdominal cavity (left) Areas where fluid usually accumulates (right) a) a - Subhepatic space,
b) b - Subphrenic space on the right,
c) c1, c2 - Subphrenic space on the left,
d) d - Paracolic space on the left,
e) e - Douglas space.
Consequences of increasing intra-abdominal pressure:
1. At values <1.33 kPa (100 mm Hg = 13.6 cm H2O), blood pressure and cardiac output are within normal limits, but visceral blood flow is significantly reduced.
2. At 1.99 kPa (15 mm Hg = 20.4 cm H2O) cardiovascular changes already occur.
3. Renal dysfunction and oliguria may occur at values> 2.66 kPa (20 mm Hg = 27.2 cm H2O).
4. Anuria occurs at values> 5.32 kPa (40 mm Hg = 54.4 cm H2O). Abdominal compartment syndrome is characterized by increased inspiratory pressures, decreased cardiac output, oliguria, despite normal or increased cardiac pulmonary pressure. Clinically significant intra-abdominal hypertension is observed in many conditions such as e.g., in postoperative intra-abdominal bleeding, in complicated intra-abdominal vascular surgery, in liver transplantation operations, in advanced diffuse peritonitis, in severe acute pancreatitis, in severe abdominal trauma, but also in peritoneal insufflation during laparoscopic patients and in laparoscopic patients. with liver cirrhosis [14]. Properly placed drain drains fluid from the abdominal cavity either passively (after a slope, with the participation of intra-abdominal pressure) or active. In any case, it is necessary to guide the drain as short as possible from the drained bearing to the body surface. Therefore, some authors recommend first placing the drain in the drained area (cavities, bearings) and only then take the drain out of the surgical wound, in the place where the drain itself is placed on the abdominal wall [15]. Targeted is the drainage of a certain demarcated area, where any fluid (bile, blood, pancreatic juice, etc.) is drained.
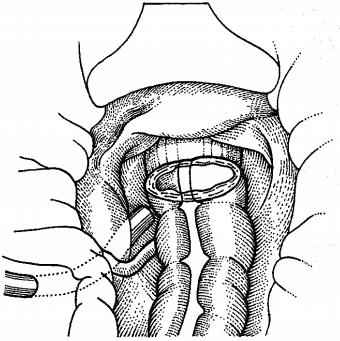
Drains established near anastomoses can be the first to inform us about possible suture insufficiency - signal drains. Active drainage is usually not recommended near anastomoses. The most commonly used closed drainage system in visceral surgery is Robinson drainage (Figure 4), which consists of a 20F silicone hose with a length of one meter. The end, which is placed in the abdominal cavity, has several holes on the sides and the opposite end is connected to a plastic calibrated bag with a volume of 350 ml. This system is modern and highly hygienic, it also contains a shut-off valve that does not allow ascending infection [16]. Infectious complications associated with bacterial contamination of the drain are one of the most important risk factors for intraabdominal drainage. Gastric surgery, such as early suture and ulcer sealing, gastric resection BI or BII, vagotomy, require drainage of the operating room. If a drain is laid, then it usually slopes into the subhepatic space or Redon’s drain (Figure 5).
Based on a German questionnaire survey, Böhm found out that 90% of them would introduce drainage after gastrectomy. Drains are most often established in the area of anastomoses, subhepatic space or in the area of the duodenal stump. The average length of drainage was 5.5 days. The suture of the ulcer was always sealed with a suture [17]. Drainage of the duodenal stump area depends on the anatomical conditions, the type of disease, the complexity of the resection and the type of duodenal stump closure. If the operating surgeon is in doubt, then he drains the duodenal stump area always sufficiently and effectively. The prognosis of early dehiscence of the duodenal stump is a very serious complication with high lethality - up to 50% [18]. After the patient’s condition improves, the fistula usually heals spontaneously. The abdominal cavity is still secured by laying a slope drain under the liver.
Use of Drainage in Small and Colon Surgery
In simple resections of the small intestine (Meckel’s divertuculus), drains are usually not established. In the case of acute small bowel surgery, e.g., due to Crohn’s disease, then it always drains. The most common indications for surgical treatment in Crohn’s disease are complications in terms of fistulations, especially in the ileocecal region [19]. Abdominal drainage after colorectal operations depends on the type of operation (acute, elective) and the scope of the operation. It is not recommended that the drain lean against the intestinal wall (Figure 6). The abdominal cavity drains only in cases of major damage to the serous surface of the intestinal wall or exposure of the lymphatic system in the retroperitoneum. In these cases, drainage is established in the area after the resected colon ascendens and/or in the Douglas space, usually for 48-72 hours. Drainage is always recommended for operations on the left half of the colon and rectum, even if no positive effects of prophylactic drainage after colonic elective procedures have been found in control studies [20]. In the case of rectal amputations, it is advantageous to introduce Redon drainage into the resulting cavity through the perineum. Usually, two drains are introduced. Böhm and co-workers report that 90% of surgeries in Germany drain the abdominal cavity after resections of the esophageal loop. They usually drain the Douglas area (54%) or the anastomosis area (31%). The average length of drainage was 5.6 days [17].
Figure 6: Drain position to intestinal suture –
a) Incorrect,
b) Correct (drain should not insist on anastomosis).
After rectal resection, it is recommended to drain the anastomosis area. This is possible either by the retroperitoneal or peritoneal route (Figure 7). In this case, the drains perform a signal function. Some literature data emphasize the importance of purging the presacral space after rectal surgery. After rectal extirpation, the perineum is actively drained using Redon’s drains. Larger calibers are chosen - usually No. 12. It is led out of the perineum forward so that the patient does not lie on them. Miles initially closed the perineal wound primarily. Active drainage was not yet known, and ascending infections arose from the catchment drainage. Miles solved this by introducing open aftercare in the form of so-called “packing” - longs placed in a thin foil. The healing process was very lengthy. Secondary healing is always very unpleasant for the patient. Today, this method is no longer used, and after rectal extirpation, primary closure of the perineal wound is always indicated. Active drainage is started at the end of the operation.
Drainage in the Pancreas
After resection operations on the pancreas, gravity drains stored in the environment are used. Surgery is most often indicated in acute pancreatitis due to signs of peritoneal irritation or septic condition. If the patient is operated on due to the edematous form of pancreatitis, bile duct remediation with possible decompression and drainage of the omental bursa is usually sufficient. In the necrotic form of acute pancreatitis, we perform neurectomy and thoroughly drain the area after the slope [21]. In these cases, drains with a wider diameter are used to prevent their early clogging by necrotic masses. It is also possible to establish a flush lavage exchange.
Individual Types of Drainages Used in the Intra- Abdominal Inflammatory Process
Percutaneous Drainage
Percutaneous drainage is inserted under CT control or ultrasound [22]. The most common drained deposits are abscesses in the area of peritoneal cavities, intra-parenchymal deposits (liver and pancreas abscess), or retroperitoneal abscesses. Other puncture lesions are most often cysts or pseudocysts of the pancreas. The best results can be achieved by evacuating well-defined small deposits with a low viscosity content. Contraindications to percutaneous drainage include cystic or necrotic tumors, abscesses around foreign bodies, abscesses associated with intestinal fistulation. The trocar technique means direct puncture with a drainage set after the previous targeting. It is always necessary to send a sample for bacteriological and cytological examination. For secretions of lower content, drainage with a lumen of 8-10 F is sufficient, for denser secretions of 10-14 F, exceptionally, a lumen of 16-18 F is used. it is necessary to monitor the amount of secretion, check the correct introduction of the drain into the drained deposit, or the amount of residual secretion in it (CT, ultrasound, sciascopy). With the correct technique of percutaneous drainage, the efficiency is in the range of 80-90%, lethality up to 1%.
Laparoscopic Remediation of An Inflammatory Deposit with Standard Drainage of the Abdominal Cavity
In some workplaces, for example, gastroduodenal ulceration with peritonitis, small pelvic abscess, tutorial abscess, resp. pelviperitonitis. We can also treat perforating appendicitis in this way. Proponents of laparoscopy say that perioperative lavage is more effective in laparoscopy than in laparotomy surgery if the abdominal fold is better defined. The main advantage of this method is the minimal trauma of the abdominal wall, there are no possible complications of a severe infection, organ emergencies, etc. [23].
Closed Continuous Peritoneal Lavage
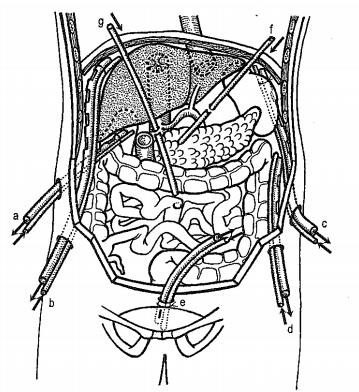
The authors of this method (Beger, McKenna) are based on the concept of peritoneal dialysis [24]. Enclosed continuous peritoneal drainage is shown in (Figure 8). They involve continuous cleansing of the cavity of toxins, blood residues, bile, or other secretions, including active enzymes. Repeated laparotomy is usually not necessary. The disadvantages are mainly the loss of proteins (especially albumin - about 1 g / l solution) and electrolytes, we cannot prevent drainage and obliteration of the drainage spaces with the risk of the formation of limited deposits of residual infection. Indications for this type of drainage include diffuse peritonitis, locally large abscesses (over 0.5l) and non-controlling pancreatitis. Standard laparotomy, removal of necrosis, evacuation of purulent deposits, and lavage of the area are followed by insertion of two to four thin catheters into the inflammatory area or fluid collection. From this area, the fluid is then drained through four to six (according to some authors up to 11) thick drains placed on the base of the abdominal cavities or directly into the area of inflammation (e.g., peripancreatically). It is advantageous to use two-way drains (Tenckhoff), due to the current lavage and drainage. Continuous lavage in the abraded cavity with a volume of about 1 liter per hour is performed with a solution intended for peritoneal dialysis.
Figure 8: Closed continuous peritoneal lavage - drainage areas in both directions
a. Subhepatic space,
b. Subphrenic space on the right,
c. Subphrenic space on the left,
d. Pericolic space on the left.
e. Douglas space,
f. Drain to the gastric major of the stomach,
g. Drain to the radix of the mesentery.
Conclusion
Drainage systems are a common part of postoperative surgical management and are used to remove fluid collection from the abdominal cavity. The drain may be superficial in the subcutaneous tissue, or deep in the organ or cavity. The number of drains depends on the scope and type of operations. Intraabdominal drainage improves early detection of complications (gastrointestinal leakage, bleeding, bile leakage), prevents fluid or pus accumulation, reduces morbidity and mortality, and shortens hospital stay. The aim of this review was to evaluate the evidence supporting the systematic use of abdominal drainage. Abdominal drainage is not easy, especially due to more complicated anatomical conditions and the presence of consciousness and intestinal loops. Very quickly after the insertion of the drain, they envelop the drain and sometimes make it functional. This is even more true for abdominal vacuum drainage. Fluid (this also applies to the abscess) tends to accumulate in the abdominal cavity at certain predilection sites (Douglas space, retroperitoneal space, subphrenic spaces, subhepatic space, paracolic spaces - depending on the patient’s position), so the surgeon’s decision to drain is in place and most often applies to these areas. Tubular drains made of biocompatible inert material are most suitable for abdominal drainage. Their location has drainage success, especially when stored in preformed areas or near parenchymal organs, to minimize the possibility of clogging with momentum or intestinal loops. Due to the nature of the effusion that is resolved by abdominal drainage, the expected drainage fluid content, and other factors, in addition to the simple tubular drain after abdominal surgery, other drainage systems are used, as mentioned above.
References
- Ramesh BA, Jayalakshmi BK (2021) Suction Drains. StatPearls. Treasure Island (FL): StatPearls Publishing.
- Puleo FJ, Mishra N, Hall JF (2013) Use of intra-abdominal drains. Clinics in Colon and Rectal Surgery 26(3): 174–177.
- Durai R, Mownah A, Philip CH N (2009) Use of Drains in Surgery: A Review. Journal of Perioperative Practice 19(6): 180-186.
- Chestovich PJ, Jennings CS, Fraser DR, Ingalls NK, Morrissey SL, et al. (2020) Too Big, Too Small or Just Right? Why the 28 French Chest Tube Is the Best Size. J Surg Res 256: 338-344.
- Ostadfar A (2016) Chapter 1 - Fluid Mechanics and Biofluids Principles. Biofluid Mechanics, p. 1-60.
- Smith SR, Connolly JC, Crane PW, Gilmore OJ (1982) The effect of surgical drainage materials on colonic healing. Br J Surg 69: 153-155.
- Černý J (1992) A kolektív: Špeciálna chirurgia II.-deli, Chirurgia brušných orgánov a retroperitonea, I. vydanie, Martin, Dérerova zbierka, Osveta, pp. 105-779.
- Čapov I, Weschler J (2001) Drény a jejich využití v chirurgických oborech. Czech : 1. Vyd.
- Čihak, Radomír a Miloš GRIM (2013) Anatomie. 2. 3. vydání. Praha : Grada.
- Pavel Petrovický (2001) Anatomie s topografií a klinickými aplikacemi (I. svazek). Martin. Osveta, pp. 463.
- Newman RK, Dayal N, Dominique E (2021) Abdominal Compartment Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing 2022.
- Schein M, Wittmann DH, Aprahamian CC, Condon RE (1995) The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg 180(6): 745-753.
- Papavramidis TS, Marinis AD, Pliakos I, Kesisoglou I, Papavramidou N (2011) Abdominal compartment syndrome - Intra-abdominal hypertension: Defining, diagnosing, and managing. Journal of Emergencies, Trauma, and Shock 4(2): 279291.
- Carlotti AP, Carvalho WB (2009) Abdominal compartment syndrome: A review. Pediatr Crit Care Med 10(1): 115-120.
- Domínguez Fernández E, Post S (2003) Abdominal drainages. Chirurg 74(2): 91-98.
- Durai R, Mownah A, Philip CH Ng (2009) Use of drains in surgery: a review. Journal of Perioperative Practice 19: 180-186.
- Böhm B, Neudecker J, Ruff J, Műller JM (1998) Zuganswege und Drainagen bei konventioneller Cholezystektomie, Gastrektomie und Sigmaresektion. Ergebnisse einer Umfrage an deutchen Kliniken. Viszeralchirurgie 33: 316-324.
- Cozzaglio L, Cimino M, Mauri G, Ardito A, Pedicini V, et al. (2011) Percutaneous transhepatic biliary drainage and occlusion balloon in the management of duodenal stump fistula. J Gastrointest Surg 15(11): 1977-1981.
- Richards RJ (2011) Management of abdominal and pelvic abscess in Crohn’s disease. World journal of gastrointestinal endoscopy 3(11): 209-212.
- Thiede A, Engemann R, Imhof M (1993) Drainagetechniken und Drainageprinzipien in der visceralen Chirurgie, Chirurg 64: 90-95.
- Beattie G, A Siriwardena A (2000) Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. British Journal of Surgery 87(2): 1020-1024.
- De Filippo M, Puglisi S, D'Amuri F, Gentili F, Paladini I, et al. (2021) CT-guided percutaneous drainage of abdominopelvic collections: a pictorial essay. La Radiologia medica 126(12): 1561-1570.
- Malkov IS, Shaĭmardanov R Sh, Zaĭnutdinov AM, Birial'tsev VN, Lustina NI (2002) Laparoscopic sanitation of the abdominal cavity in combined treatment of peritonitis. Khirurgiia (Mosk) (6): 30-33.
- Beger HG, Isenmann R (1999) Surgical management of necrotising pancreatitis. Surg Clin North Am 79(4): 783-800.

 Review Article
Review Article