ABSTRACT
The definition of diabetic ketoacidosis is biochemically expressed as venous potential hydrogen 200mg/dL (11mmol/L) together with ketonemia, glucosuria, and ketonuria. Several conditions can lead to the development of diabetic ketoacidosis such as infections; new diagnosis of diabetes; poor adherence to insulin, or inadequate doses of insulin. Diabetic ketoacidosis is the result of a critical relative or absolute deficiency of insulin, resulting in intracellular starvation of insulin-dependent tissues (muscle, liver, adipose), stimulating the release of the counter-regulatory hormones such as glucagon, catecholamines, cortisol, and growth hormone. The intentions of diabetic ketoacidosis management with fluid and insulin is to restore perfusion, which will elevate glucose uptake in the periphery, elevate glomerular filtration, and reverse the progressive acidosis; arrest ketogenesis with insulin administration, which reverses proteolysis and lipolysis while stimulating glucose uptake and processing, thereby normalizing blood glucose concentration. Intravenous fluid replacement is begun as soon as the diagnosis of diabetic ketoacidosis is established. Initial fluid resuscitation begins with 10mL/kg of isotonic fluid, either 0.9% saline or lactated ringer solutions, administered over 1hr. The administration of insulin 0.1unit/kg subcutaneously every hr may be preferable and can be adjusted to maintain blood glucose concentrations at approximately 180-200mg/dL (10-11mmol/L).
Keywords: Diabetic Ketoacidosis; Pathophysiology; Precipitating Factors; Management
Abbreviations: DM: Diabetes Mellitus; DKA: Diabetic Ketoacidosis; KCL: Potassium Chloride; NAHCO3: Sodium Bicarbonate; T2DM: Type-1 Diabetes Mellitus
Introduction
Diabetes mellitus can be described as a chronic disease occurred due to elevated blood sugar level because of the body cannot produce insulin at all or secrets insufficient insulin hormone or not use it effectively. The nonexistence of insulin or the cell is not sensitive to use insulin leads to enhanced blood glucose level which is the hallmark of diabetes mellitus. Diabetes mellitus affects more than 422 million people around the world. By the year 2040, the number of people with diabetes is expected to rise to 642 million, most of who are going to reside in low- or middle-income countries. Diabetes mellitus is a growing public health problem affecting people worldwide, with a rapidly elevating prevalence in both advancing and advanced countries [1]. The World Health Organization observed that high blood sugar level due to diabetes is the third highest risk factor for premature mortality after high blood pressure and tobacco use [2]. T1DM can be a common autoimmune condition that often presents in childhood and perhaps complicated by episodes of diabetic ketoacidosis [3]. One of the alarming life-threatening complications of type 1 diabetes mellitus is diabetic ketoacidosis [4]. The definition of diabetic ketoacidosis is biochemically expressed as venous potential hydrogen 200mg/ dL (11mmol/L) together with ketonemia, glucosuria, and ketonuria. DKA may be rarely occurring with normal circulating glucose concentrations; if there has been partial management or with pregnancy. The severity of DKA is determined by the degree of acidosis such as mild; when venous pH >7.2 and <7.3, bicarbonate <15 mmol/L; moderate; when venous pH >7.1 and <7.2, bicarbonate <10 mmol/L; severe when venous pH <7.1, bicarbonate <5 mmol/L [5]. Diabetic ketoacidosis is a life threatening emergency manifesting with hyperglycemia when random blood sugar >200 mg/dL, high anion gap metabolic acidosis (pH-3) [6].
Precipitating Factors of DKA
Patients with diabetes mellitus who are admitted with diabetic ketoacidosis should be counselled about the precipitating cause and early warning symptoms. Failure to do so is a missed educational opportunity. Things to consider include the following
i. Identification of precipitating factors such as infection or omission of insulin injections;
ii. Education to prevent recurrence; for example, provision of written sick day rules;
iii. Warning about potential insulin ineffectiveness; for example, the patient’s insulin may be expired or denatured;
iv. Provision of hand-held ketone meters with education on management of ketonaemia [7-11]. Several conditions can lead to the advancement of DKA such as infections; new diagnosis of diabetes; poor adherence to, or inadequate doses of, insulin; myocardial infarction; stroke; acute pancreatitis; trauma; burns; surgery; medications such as glucocorticoids, beta blockers, thiazides and atypical antipsychotics; psychological factors including depression and eating disorders and illicit substance use [7,12].
Pathophysiology of DKA
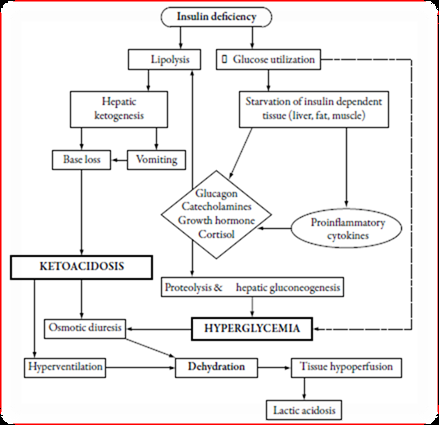
DKA is the result of a critical relative or absolute deficiency of insulin, resulting in intracellular starvation of insulin-dependent tissues (muscle, liver, adipose), stimulating the release of the counter-regulatory hormones glucagon, catecholamines, cortisol, and growth hormone. The counter-regulatory hormonal responses may also be the result of stress-induced proinflammatory cytokines [5]. Hyperglycemia and ketosis in diabetic ketoacidosis are the result of insulin deficiency and elevate in the counterregulatory hormones glucagon, catecholamines, cortisol, and growth hormone. Three processes are mainly responsible for hyperglycemia such as elevated gluconeogenesis, accelerated glycogenolysis, and impaired glucose utilization by peripheral tissues [13]. Insulin deficiency, elevated insulin counter-regulatory hormones (cortisol, glucagon, growth hormone, and catecholamines), and peripheral insulin resistance lead to hyperglycemia, dehydration, ketosis, and electrolyte imbalance, is the underlie pathophysiology of DKA. Due to elevated lipolysis and decreased lipogenesis, abundant free fatty acids are converted to ketone bodies including β-hydroxybutyrate (β-OHB) and acetoacetate. Hyperglycemia-induced osmotic diuresis, if not accompanied by sufficient oral fluid uptake, leads to dehydration, hyperosmolarity, electrolyte loss, and subsequent lower in glomerular filtration rate. With decline in a renal function, glycosuria diminishes and hyperglycemia worsens. With impaired insulin action and hyperosmolar hyperglycemia, potassium uptake by skeletal muscle is markedly diminished; also hyperosmolarity can cause efflux of potassium from cells. This results in intracellular potassium depletion and subsequent loss of potassium via osmotic diuresis, causing reduction of total body potassium averaging 3-5mmol/kg of body weight. A “normal” plasma potassium concentration still indicates that total body potassium stores are severely diminished, and the institution of insulin therapy and correction of hyperglycemia will result in hypokalemia [14,15] (Figure 1).
Treatment of DKA
The intentions of management of DKA with fluid and insulin is to restore perfusion, which will elevate glucose uptake in the periphery, elevate glomerular filtration, and reverse the progressive acidosis; arrest ketogenesis with insulin administration, which reverses proteolysis and lipolysis while stimulating glucose intake and processing, thereby normalizing blood glucose concentration; replace electrolyte losses [5]. Several significant steps should be followed in the early stages of DKA management are
1) Collect blood for metabolic profile before initiation of intravenous fluids;
2) Infuse 1L of 0.9% sodium chloride over hr after drawing initial blood samples;
3) Ensure potassium level of 3.3 mEq/L before initiation of insulin therapy;
4) Initiate insulin therapy only when steps 1-3 are executed [14]. Management of DKA consists of fluid and electrolyte replacement, insulin administration, and careful ongoing monitoring of clinical and laboratory factors.
Fluid and Electrolyte Replacement: The osmotic diuresis generated by glucosuria results in large water and electrolyte losses, exacerbated by compromised uptake due to nausea and vomiting. Initial fluid resuscitation begins with 10mL/kg of isotonic fluid, either 0.9% saline or lactated ringer solution, administered over 1hr. For more critically ill pediatric, for whom there is concern over impending cardiovascular collapse, additional resuscitation fluid should be administered more quickly. After the initial fluid resuscitation, the remainder of the fluid deficiency is replaced evenly over 48hrs. Most patients who have DKA are approximately 6% dehydrated and 10% for 2 yrs pediatric. For patients presenting with more severe DKA (serum glucose 600 to 800 mg/dL (33.3 to 44.4mmol/L) and pH 7.1)), fluid losses are approximately 9% of body weight and 15% for 2yrs pediatric. The 0.9% saline (with added potassium) is continued as the hydration fluid until the blood sugar value declines to less than 300mg/dL (16.7mmol/L) and at that time, our practice is to change the fluid to D5 0.45% saline (with added potassium). The American Diabetes Association recommendation is that deficit replacement fluids contain at least 0.45% saline with added potassium. If the blood glucose concentration declines below 150mg/dL (8.3mmol/L) the dextrose content may need to be elevated to 10% or even 12.5%. Both insulin managements of DKA and correction of the acidosis cause potassium to move intracellularly. Unless the patient exhibits hyperkalemia or anuria, potassium should be added to the intravenous fluids at the beginning of the second hr of treatment. If the patient presents with hypokalemia, potassium replacement is initiated immediately. Most patients require 30 to 40mEq/L of potassium in the replacement fluids, with adjustment based on serum potassium concentrations that are measured every 1 to 2hrs. DKA results in significant phosphate depletion, and serum phosphate values decrease during treatment.
Hypophosphatemia may cause metabolic disturbances. Phosphate replacement should be given if the values of phosphate decrease below 1mg/dL. In the absence of severe hypophosphatemia, provide phosphate in intravenous fluids, typically by giving half of the potassium replacement as potassium phosphate. Administration of potassium acetate to provide the other half of the potassium replacement further decreases the chloride load. The serum calcium concentration must be monitored if phosphorus is given, due to the risk of hypocalcemia. If hypocalcemia develops, phosphate administration should be stopped. During the treatment of DKA, the patient can produce substantial bicarbonate as insulin stimulates the generation of bicarbonate from the metabolism of ketones. Potential risks of bicarbonate treatment involve paradoxic central nervous system acidosis and exacerbation of hypokalemia. Bicarbonate management also has been correlated with cerebral edema, the most frequent cause of mortality for children who have DKA. Therefore, bicarbonate treatment should be considered only in cases of extreme acidosis, such as for the patient whose pH is 6.9, when the acidosis may impair cardiovascular stability, or as management of life-threatening hyperkalemia. If bicarbonate administration is believed to be necessary, 1 to 2mmol/kg (added to 0.45% saline) should be provided over 1 to 2hrs [15].
Insulin Therapy: Insulin should be started after initial fluid expansion and provides a more realistic starting glucose level 0.1U/kg/hr is given as a continuous infusion, using a pump. 50Us of regular insulin are diluted in 50mL normal saline to provide 1unit/ mL. The administration of 0.1unit/kg subcutaneously every hr perhaps preferable and can be adjusted to maintain blood glucose concentrations at approximately 180-200mg/dL (10-11mmol/L). Fluid expansion alone will have a dilutional effect, lowering high blood glucose levels by as much as 180-270mg/dL (10-15mmol/L). With insulin infusion the rate of glucose decline should be 50- 150mg/dL (2.8-8.3mmol/L/hr), but not >200mg/dL (11mmol/L/ hr). If the blood sugar concentration falls below 150mg/dL (8.3mmol/L) 10% dextrose solution should be given and the insulin dose reduced to 0.05 U/kg/hour if a glucose concentration is not sustained by the 10% dextrose solution. Insulin should not be stopped; a continuous supply of insulin is needed to inhibit ketosis and permit continued anabolism. If the individuals demonstrate marked sensitivity to insulin, the dose may be lowered to 0.05units/ kg/hour, or less, provided that metabolic acidosis continues to resolve [5,16-18].
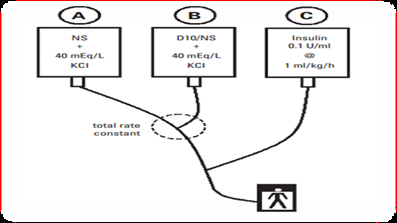
The Two-Bag System: Once the patient is receiving fluids and then insulin, the blood glucose will fall, usually quite rapidly. The objective of two bag system is to maintain the blood glucose in the 10 to 15mmol/L range over the first day or so, to provide a buffer against the advancement of hypoglycemia. Two bags of intravenous fluids, similar in their electrolyte composition and differing only in their dextrose concentration, are run in parallel through the same cannula. The total fluid rate from these two bags determined by the protocol will be constant, and the final concentration of dextrose can be altered simply by juggling the rates of the two bags. The two-bag system is easy to institute, uses commercially available solutions, and has been revealed to reduce the time needed to make an alter in IV rates, to lower the number of IV bags used during an admission, and to reduce the cost of IV solutions used [19,20] (Figure 2).
Intravenous Glucose Infusion: Management of DKA should be aimed on clearing ketones as well as normalizing blood sugar. Introduction of 10% glucose is recommended when the blood glucose falls below 14mmol ⁄ l in order to avoid hypoglycemia, while continuing the fixed-rate intravenous insulin infusion to prevent ketogenesis. It is significant to continue 0.9% sodium chloride solution coincidently to correct circulatory volume if the fluid deficit has not been corrected. Glucose should not be discontinued until the patient is eating and drinking normally [21].
Potassium Therapy: Adults with DKA have total body potassium deficits of the order of 3-6mmol/kg. The major loss of potassium is from the intracellular pool as a result of hypertonicity, insulin deficiency, and buffering of hydrogen ions within the cell. Serum potassium levels at the time of presentation may be normal, elevated or lowered hypokalemia at presentation perhaps related to prolonged duration of disease, whereas hyperkaliemia primarily results from lowered renal function. Administration of insulin and the correction of acidosis will drive potassium back into the cells, lowering serum levels [22].
Bicarbonate Therapy: Alkali therapy in DKA has not been routinely recommended, as metabolic derangements tend to correct with insulin therapy and fluids as hypovolemia, tissue perfusion and renal function improve. As a consequence of the elevated severity of metabolic acidosis with pH less than 7.0, bicarbonate may empirically be given as an isotonic solution with an initial dose of 50mmol intravenous bicarbonate (one ampoule of 7.5 % NaHCO3 solution in 250ml sterile water) with 15mEq of KCL for each ampoule of bicarbonate administered if serum potassium 5.5mEq/L. Alternatively, if the pH is 6.9, 100mmol (100mEq) administered in 400mL sterile water may be infused at 200mL/h with frequent re-dosing every 2hrs until pH exceeds 7 [23-26].
Phosphate Therapy: Whole-body phosphate depletion is a hallmark of poorly controlled diabetes mellitus. Hyperglycemia and hyperosmolarity cause an intracellular to extracellular shift of serum phosphate; due to this reason, serum phosphate levels may be normal or increased at the onset of DKA. Insulin therapy in the setting of DKA perhaps show hypophosphatemia as insulin drives phosphate back into cells. Potassium or sodium phosphate supplementation (20-30mEq/L) may be added to replacement fluids over several hrs with close monitoring of serum calcium and phosphate levels. Alternatively, in patient tolerating oral intake with mild deficits, oral phosphate (2.5-3.5 g/day in 2-3 divided doses may be administered [23,27,28].
Conclusion
Diabetic ketoacidosis is a life threatening emergency manifesting with hyperglycemia when random blood sugar >200mg/dL, high anion gap metabolic acidosis (pH-3). Several conditions can lead to the advancement of DKA such as infections; new diagnosis of diabetes; poor adherence to, or inadequate doses of, insulin; myocardial infarction; stroke; acute pancreatitis; trauma; burns; surgery; medications such as glucocorticoids, beta blockers, thiazides and atypical antipsychotics; psychological factors including depression and eating disorders and illicit substance use. The counter-regulatory hormonal responses may also be the result of stress-induced proinflammatory cytokines. Therapy of diabetic ketoacidosis consists of fluid and electrolyte replacement, insulin administration, and careful ongoing monitoring of clinical and laboratory factors.
Acknowledgment
The author extends his gratitude to those all who support him amid manuscript preparation by bestowing constructive information.
Data Sources
Sources searched include Google Scholar, Research Gate, PubMed, NCBI, NDSS, PMID, PMCID, and Cochrane database. Search terms included: diabetic ketoacidosis
Funding
None.
References
- Bereda G (2021) Brief overview of diabetes mellitus. Diabetes Manag 11(S1): 21-27.
- Bereda G, Bereda G (2021) The Incidence and Predictors of Poor Glycemic Control among Adults with Type 2 Diabetes Mellitus in Ambulatory Clinic of Mettu Karl Referral Hospital, South Western, Ethiopia: A Prospective Cross Sectional Study. Int Arch Endocrinol Clin Res 7: 024.
- Foster JR (2011) Diabetic Ketoacidosis-Associated Stroke in Children and Youth. Stroke Research and Treatment 219706: 12.
- Sehgal V, Ulmer B (2019) Clinical conundrums in the management of diabetic ketoacidosis in the elderly. J Transl Int Med 7(1): 10-14.
- Rosenbloom Arlan L (2010) The Management of Diabetic Ketoacidosis in Children. Diabetes Ther 1(2): 103-120.
- Ravikumar N, Bansal A (2021) Application of bench studies at the bedside to improve outcomes in the management of severe diabetic ketoacidosis in children-a narrative review. Transl Pediatr 10(10): 2792-2798.
- MW Savage, Ketan Dhatariya, A Kilvert, Gerry Rayman, JAE Rees, et al. (2011) Diabetes UK Position Statements and Care Recommendations. Diabet Med 28: 508-515.
- Bektas F, Eray O, Sari R, Akbas H (2004) Point of care testing of diabetic patients in the emergency department. Endocr Res 30(3): 395- 402.
- Khan ASA, Talbot JA, Tiezen KL, Gardener EA, Gibson JM, et al. (2004) Evaluation of a bedside blood ketone sensor: the effects of acidosis, hyperglycaemia and acetoacetate on sensor performance. Diabet Med 21(7): 782-785.
- Wallace TM, Matthews DR (2004) Recent advances in the monitoring and management of diabetic ketoacidosis. Q J Med 97(12): 773-780.
- Vanelli M, Chiari G, Capuano C, Iovani B, Bernardini A, et al. (2003) The direct measurement of 3-beta-hydroxy butyrate enhances the management of diabetic ketoacidosis in children and reduces time and costs of treatment. Diabetes Nutr Metab 16(5-6): 312-316.
- Sheikj Ali M, Karon BS, Basu A, Kudva YC, Muller LA, et al. (2008) Can serum beta-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care 31(4): 643-647.
- Thawabi M, Studyvin S (2015) Euglycemic diabetic ketoacidosis, a misleading presentation of diabetic ketoacidosis. North Am J Med Sci 7(6): 291-294.
- Gosmanov, Elvira O Gosmanova, Erika Dillard Cannon (2014) Management of adult diabetic ketoacidosis. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 7: 255-264.
- David W, Leslie Plotnick (2008) Cooke and Leslie Plotnick. Management of Diabetic Ketoacidosis in Children and Adolescents. Pediatr Rev 29(12): 431-436.
- Edge J, Jakes R, Roy Y, M Hawkins, D Winter, et al. (2006) The UK case control study of cerebral oedema complicating diabetic ketoacidosis in children. Diabetologia 49(9): 2002-2009.
- Umpierrez GE, Cuervo R, Karabell A, Latif K, Freire AX, et al. (2004) Treatment of diabetic ketoacidosis with subcutaneous insulin aspart. Diabetes Care 27(8): 1873-1878.
- Della Manna T, Steinmetz L, Campos PR, Sylvia C L Farhat, Cláudio Schvartsman, et al. (2005) subcutaneous use of a fast-acting insulin analog: an alternative treatment for pediatric patients with diabetic ketoacidosis. Diabetes Care 28(8): 1856-1861.
- Daniel L Metzger (2010) Diabetic ketoacidosis in children and adolescents: An update and revised treatment protocol. BC Medical Journal 52(1): 24-31.
- (2009) BC Children’s Hospital Endocrinology and Diabetes Unit. Diabetic ketoacidosis protocol toolkit.
- Eledrisi MS, Elzouki AN (2020) Management of diabetic ketoacidosis in adults: A narrative review. Saudi J Med Med Sci 8(3): 165-173.
- DB Dunger, MA Sperling, CL Acerini, DJ Bohn, D Daneman, et al. (2004) ESPE/LWPES consensus statement on diabetic ketoacidosis in children and adolescents. Arch Dis Child 89(2): 188-194.
- Perilli G, Christine Saraceni, Michael N Daniels, Aakif Ahmad (2013) Diabetic Ketoacidosis: A Review and Update. Curr Emerg Hosp Med Rep 1: 10-17.
- Eledrisi MS, Alshanti MS, Shah F, Basem Brolosy, Nermeen Jaha (2006) Overview of the diagnosis and management of diabetic ketoacidosis. Am J Med Sci 331(5): 243-251.
- Kitabchi AE, Umpierrez GE, Fisher JN, Mary Beth Murphy, Frankie B Stentz (2008) Thirty years of personal experience in hyperglycemic crises: diabetic ketoacidosis and hyperglycemic hyperosmolar state. J Clin Endocrinol Metab 93(5): 1541-1552.
- Kitabchi AE, Umpierrez GE, Murphy MB, EJ Barrett, RA Kreisberg, et al. (2001) Management of hyperglycemia crises in patients with diabetes. Diabetes Care 24(1): 131-153.
- Chaithongdi N, Subauste J, Koch C, Geraci S (2011) Diagnosis and management of hyperglycemic emergencies. Hormones 10(4): 250-260.
- Abbas E Kitabchi, Guillermo E Umpierrez, Mary Beth Murphy, Eugene J Barrett, Robert A Kreisberg, et al. (2004) Hyperglycemia crises in diabetes. Diabetes Care 27(1): S94-102.

 Review Article
Review Article