ABSTRACT
In modern medical science, targeting the tumour vasculature instead of the tumour cells is of great interest for the management of tumour associated complications. In context to that, the review was planned to explore the mechanisms of pathological angiogenesis responsible for cancer cell invasion and metastasis. The experimentally proven phytopharmaceuticals having a significant effect to inhibit vasculature development are represented schematically. The scientific literature from authenticated databases (Scopus, PubMed, etc.) search was conducted with emphasis on the previous fifteen years, combining the keywords as selected. Mechanisms of pathological angiogenesis responsible for cancer cell invasion and metastasis have been explained with possible regulatory protein involvement. A total of 97 experimentally proven plant molecules, studied in this review, including 69 plant species among 40 plant families, are summarized in a schematic way.
Hopefully, this review will facilitate the biomedical scientists in setting up the appropriate research questions around the molecular targets discussed in this review for the management of cancer cell invasion and migration and for further proof-ofconcept validation studies for exploring such phytopharmaceuticals.
Keywords: Pathological Angiogenesis; Cancer Cell Invasion; Metastasis; Cell Migration; Experimentally Proven Phytopharmaceutical; Inhibition of Vasculature Development; Modern Target for Cancer treatment
Introduction
The formation of new blood vessels is a vital multistep process in our body with both advantages and disadvantages, as it is responsible for the normal physiological growth on one hand while on the other it accounts for some diseases [1]. Blood vessels aid in oxygen and essential nutrients delivery to the cells and discard catabolic wastes from them [2]. The formation of new blood vessels from a pre-existing one is known as angiogenesis or neovascularization [3]. In 1971, a hypothesis by Judah Folkman first demonstrated that; “the growth of solid neoplasms is always accompanied by neovascularization” [4]. He also isolated a stimulatory factor, Tumour Angiogenesis Factor (TAF), present only in the tumour cells (exception: placenta) [4]. In the absence of angiogenesis, cancer cells cannot grow beyond 2mm3 and may become necrotic or apoptotic [5]. Angiogenesis initiation is triggered by various chemical or physiological factors, among which “Hypoxia” is the key inceptive factor (physiological) [6]. The initiation of angiogenesis, also referred to as “Angiogenic Switch” is a tumour growth and progression process influenced by the tumour type, its microenvironment, and other stimulatory factors, and can eventuate at any stage of a tumour [7]. There are some angiogenic stimulatory factors or pro-angiogenic factors or TAF or Tumour Angiogenesis Factors (VEGF, FGF, EGF, etc.) that assist angiogenesis and some antiangiogenic factors (Thrombospondin-1, statins, etc.) that have inhibitory effects, a gross amount of which leads to tumour dormancy, even for a few years [7].
However, when the normal proportion of pro-angiogenic and antiangiogenic attributes are imbalanced (basically proangiogenics increases largely than anti-angiogenics), angiogenesis triggers, uncontrolled vessel formation starts and the dormant tumour starts proliferating, a phenomenon is known as “Angiogenic Switch” [8]. Among the many angiogenesis affecting factors, vascular endothelial growth factor or VEGF was the first identified (1983) angiogenesis initiator and thrombospondin-1 or TSP-1 was the first identified (1990) angiogenesis inhibitor [8]. White Adipose Tissue (WAT) and Brown Adipose Tissue (BAT) are responsible for angiogenic factor production. WAT maintains vascular growth while BAT is involved with the metabolic processes of tumour growth [9]. Synergistic action of pericytes (perivascular cells that wrap around blood capillaries) and endothelial cells involves some regulators that may be responsible for physiological and pathophysiological conditions like; vasculature development, angiogenesis, and tumour metastasis [10]. Although, the role of pericytes in angiogenic sprouting is not quite clear, pericytetargeted therapies have become very effective these days, to inhibit uncontrolled tumour growth [10]. In this review, we have summarized and explained pathological angiogenesis, its role in cancer cell invasion and metastasis and presented schematically the important experimentally proven phytopharmaceuticals that have been found to be beneficial in inhibiting vasculature development. The review is expected to facilitate the biomedical scientists in setting up appropriate research questions around molecular targets for the management of cancer cell invasion and migration.
Angiogenesis and Type of Angiogenesis
Angiogenesis has a great impact on normal physiological growth as well as disease conditions. Physiological angiogenesis is associated with normal tissue growth and vasculogenesis, whereas pathological angiogenesis is associated with illness. Almost all pro-angiogenics are related to various angiogenesis types. Especially, vascular endothelial growth factor (VEGF) plays a key role in both normal angiogenesis (by ensuring endothelial cell proliferation, survival, and metastasis) as well as in angiogenic disorders or pathological angiogenesis (by enhancing the release of proinflammatory cytokines) [11]. Extracellular matrix (ECM) and vascular basement membrane (BM) are key mediators in physiological angiogenesis [12,13]. Vascular or circulatory system is the first physiological system that develops in mammalian embryogenesis [14]. Physiological angiogenesis occurs during wound healing, menstrual cycle, embryo implantation, pregnancy, etc. [15]. There are various in vitro and in silico models (continuum model, cell-based model, hybrid mathematical model) for wound healing angiogenesis, but these have some limitations which need further improvement [16]. Leutial angiogenesis, stimulated and regulated by Macrophages, Polymorphonuclear neutrophils, Eosinophils, etc., occurs almost regularly in the corpus luteum (CL) and is related to the formation and function of the luteal structure, ovulation, peripubertal and postpartum periods, etc. [17,18].
Embryo implantation is regulated by both physiological and pathological angiogenesis in the endometrium [19]. Female reproductive hormones, e.g., Estrogen, Progesterone, Human Chorionic Gonadotrophin (hCG), etc., regulate various stages of endometrium angiogenesis [20]. The imbalance of angiogenic factors during pregnancy may lead to miscarriage, defective placentation, or other pregnancy-related disorders [20]. Mitochondria are also indirectly linked with the angiogenesis process. Mitochondrial Complex III produces mROS (mitochondrial reactive oxygen species) that stabilizes HIF-1α, which then releases VEGF from cells leading to angiogenesis [21]. Skeletal muscle is also driven by the angiogenesis process [22]. Alteration of pro-angiogenics and anti-angiogenics balance leads to the shifting of physiological angiogenesis to pathological angiogenesis, thus resulting in diseased conditions, like; tumor formation and progression, all types of cancers (breast, liver, lung, ovarian, GIT, melanoma, etc.), diabetic retinopathy, cardiovascular diseases, psoriasis etc. in the body [23-24]. Pathological retinal angiogenesis is related to vascular leakage, bleeding and fibrosis, visual impairment, etc. and occurs in disease conditions like; retinopathy of prematurity (ROP) and age-related macular degeneration (AMD caused by angiogenic factor imbalance, by factors like-retinal hypoxia, ischemia or inflammation) [25]. Ocular neovascular disease, a leading cause of vision impairment and blindness, occurs because of IL-17 regulated VEGF and other inflammatory cytokines [26].
Diabetic retinopathy or DR (caused by vascular damage in the retina) is a pathophysiological condition associated with VEGF overexpression and some proinflammatory cytokines (TNF-α, IL 1β, etc.) [27]. Therapeutic angiogenesis is an experimental approach that deals with the external delivery of angiogenic growth factors (like; VEGF, HIF-1) for the treatment of ischemic or injured tissues or fibrosis to promote targeted neovascularization or surgical revascularization process [28]. HIF-1 is used to cure endometriosis and blindness, VEGF can be used (in vivo) in coronary and peripheral artery disease, ischemic ulcers, etc. [15,28]. Particular biomaterials deliver these angiogenic stimulatory factors to our body in some specific manner; example: PEG hydrogel, PEG-fibrinogen hydrogel, PEGDA hydrogel, Porcine pericardium, ECM, PLG microspheresinscaffold, etc. [12]. Gene therapy, stem cell therapy, microvesicle/ exosome therapy, combinational gene stem cell therapy, engineered exosome therapy, etc., are some well-established scientific approaches to therapeutic angiogenesis [29].
Mode of Vessel Formation and Branching
Circulatory or cardiovascular system maintains body homeostasis along with other physiological process like- supply of blood cells, essential nutrients, oxygen, and elimination of waste materials by creating a blood vessel network all over our body [30]. Blood vessel formation occurs mainly via Vasculogenesis and Angiogenesis, two different processes of vascularization consisting of various molecules and signalling pathways [30,31]. Mainly Embryonic development triggers vasculogenesis, whereas angiogenesis can be triggered by hypoxia and some other factors [31]. Vasculogenesis results in the formation of vascular network in embryonic stage followed by the expansion of those blood vessels by angiogenesis [31]
However, some factors responsible for both vasculogenesis and angiogenesis includesa.
HIF-1α stimulation by Lactate in endothelial cells (EC) under normoxic conditions leads to hypoxia whereas Lactate mediated vascular endothelial growth factor (VEGF pathway) upregulation results in vasculogenesis and tumour angiogenesis [32,33].
b. Endothelial Ca2+ signalling induces both angiogenesis and vasculogenesis [34].
c. CD27-CD70 T cell co-stimulation in lymphoid organs results in neovascularization in the human body [35].
d. Vessel branching process mainly occurs through two distinct mechanisms.
I. By bud or sprout formation of pre-existing vessels or sprouting angiogenesis and,
II. By forming of pillar or tube-like new vessels from endothelial cells or non-sprouting angiogenesis or splitting angiogenesis or intussusceptive angiogenesis [36,37].
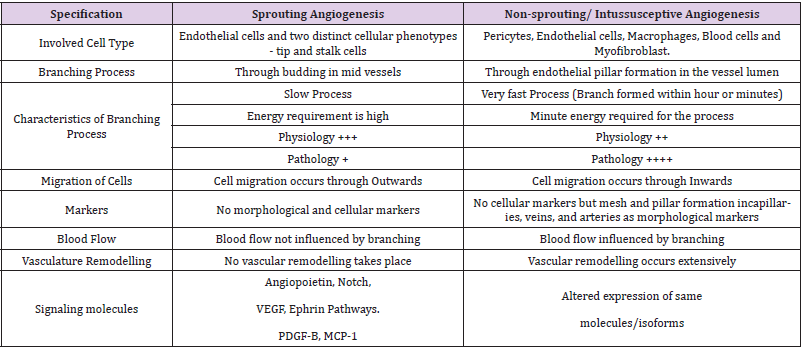
Sprouting angiogenesis (SA), mechanized with the budding process, undergoes three main steps; proliferation or dilation, elongation, stabilization, where elongation further consists of cell migration, basement membrane degradation, lumen formation [38,39]. In such angiogenesis, the endothelial cell (EC) is proliferated and initiates sprouting with the help of VEGFAngiopoietin factor [38]. Two significant cellular phenotypes, tip cells and stock cells, and some factors like platelet-derived growth factor-β (PDGF-β), matrix metalloproteinases (MMPs) play a vital role in vessel elongation and tube formation, [38,39]. The stock cells work in a proliferative way while tip cells as migratory units in lumen formation to form a vascular network [39]. Angiopoietins and their receptors (Tie-1 and Tie-2) then decrease the pericyteendothelial cell interactions and stabilize these newly formed vessels [38]. In a recent study, it has been shown that a transcription factor, myocyte enhancer factors-2 (MEF2) significantly regulated SA by upregulating Delta-like ligand-4 (Dll4) factor [40].
Meanwhile, intussusceptive angiogenesis (IA), coordinated by the ‘intussusception’ process, may be defined as the transluminal tube formation and splitting longitudinally into two vessels from a preexisting single blood vessel [41]. The term ‘intussusception’ signifies ‘growth within itself’ [42]. Mainly, eruption and branching of new vessels occur due to cytoplasmic partial pressure [38]. IA undergoes a very complicated series of process
a. Expansion of blood vessels by intussusceptive microvascular growth (IMG) mechanism,
b. Isolation of new vessels by intussusceptive arborization (IAR) mechanism,
c. Augmentation through intussusceptive branching remodelling (IBR) [43]. Due to the fast and unpredictive nature of the mechanism, the vascular network is reconstructed now and then [41].
The general interrelations between SA and IA are shown in Table 1. Although, sprouting angiogenesis is a standard antiangiogenic therapy target for treating malignant and non-malignant human diseases, intussusceptive angiogenesis (IA) is also a significant target in controlling tumour growth for some reasons.
• It is a rapid but low energy consuming branching process with approximately 50% of the total vasculature in certain types of cancers originated through IA. Therefore, it can be an important druggable target.
• IA also assists in tumour regrowth and expands the vascular network rapidly in local or organ specific, even after antiangiogenic therapy.
• Angiogenic switch plays a key role in the development of antiangiogenic therapy and tumour resistance. Angiogenic switch from SA to IA heavily depends upon some pro-angiogenic factors (NO VEGF signalling, etc.) [38].
Angiogenic Stimulatory Factors
Various mechanical, chemical or molecular factors trigger the angiogenesis process.
Mechanical Stimulatory Factors
Researchers found clear evidence on the impact of mechanical microenvironment on tumor angiogenesis, however their mechanism processes are still confusing and controversial [44,45].
However, recently it has been identified that,
I. Fluid shear stress of blood capillaries.
II. Increased muscle contraction led to the rise of NO level.
III. Increased Collagen Matrix and Endothelial-Cell-Matrix (ECM) stiffening can introduce mechanical stimulation in tissue angiogenesis [44,45].
Molecular Stimulatory Factors
A number of chemical factors and pathways also have a great impact on angiogenesis. Some of them are summarized here
VEGF: The vascular endothelial growth factor (VEGF) is the key mediator of angiogenesis along with cancer cell proliferation, invasion, and metastasis [46]. VEGF belongs to a heparin-binding glycoprotein family, consisting of VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PLGF) and shows affinity towards 3 types of receptors, VEGFR1 (binds with VEGF-A, B and PLGF), VEGFR2 (binds with VEGF-A), and VEGFR3 (binds with VEGF-C and VEGF-D) [47]. Many studies support the presence of VEGFRs in liquid and solid tumours like; NSCLC, melanoma, prostate cancer, leukaemia, breast cancer, etc. which on subsequent stimulation by VEGF regulates tumour cell proliferation [48]. VEGF shows both neurotrophic and neuroprotective effects on glial and neuronal cells; apparently, they actively participate in neuronal vessel development in CNS and PNS [49]. It is also associated with ocular neovascularization [50]. Often, VEGF-a overexpression results in Hepatocellular carcinoma and lung cancer [46,51]. VEGF and SEMA4D synergistically showed an angiogenic effect on ovarian cancer [52]. PIN2/TRF1-interacting telomerase inhibitor-1 or PinX1 can suppress renal cancer angiogenesis through downregulation of VEGF expression in the mir-125a-3p/VEGF signalling pathway [53]. PR1P, a novel therapeutic peptide, binds with VEGF, which on further overexpression promotes fibrosis or revascularization of injured tissue [54].
HIF-1: Hypoxia, a principal physiological state of our body, is caused by the unrestrained replication of cancer cells, the development of nonfunctional vasculature in solid tumoursetc. and noticed when a shortage of adequate supply of oxygen in body tissue fails to meet oxygen demand [55,56]. HIF or hypoxiainducible factor, which is a heterodimeric transcriptional factor, consists of α and β (also known as aryl hydrocarbon receptor nuclear translocator or ARNT) subunits which are further subdivided into (1) HIF-1α or HIF-1, (2) HIF-2α or HIF-2, (3) HIF- 3α or HIF-3 and (1) ARNT1, (2) ARNT2, (3) ARNT3 [55,57]. Among all, the α subunit (especially HIF-1α), is more oxygen-sensitive and the key mediator of a hypoxic response that produces angiogenic growth factors and various cytokines leading to angiogenesis [58]. HIF-1α plays a pivotal roles in cardiac hypertrophy and end-stage heart failure, whereas the development of HIF-2α by myocardial hypoxia can play a protective role in cardiac failure [58]. The HIF- 1α expression regulates every step involved in tumorigenesis towards cancer like; cell cycle regulation, glucose metabolism, angiogenesis, erythropoiesis, cell proliferation and invasion, etc., and radio resistance neovascularization by releasing the pro-angiogenic cytokine (i.e.-VEGF) although HIF-1-dependent tumour cell apoptosis has inhibitory effect on tumour growth by promoting glucose deprivation [59]. HIF-1α is also involved in pulmonary hypertension, critical limb ischaemia (CLI), retinopathy, diabetic ulcer, ageing, etc [60]. HIF-1α along with its downstream factors regulate metabolic reprogramming and angiogenesis in cutaneous tumours like; Merkel cell carcinoma, melanoma, basal cell carcinoma, and squamous cell carcinoma [61].
TNF-α: Tumour Necrosis Factor (TNF) is an inflammatory cytokine (protein), derived from monocytes, other immunological or parenchymal cells [62,63]. TNF was first reported about 45 years ago, in mid-1975, by Carswell et al.; although TNF was first observed in the 1960s [64]. Carswell et al. reported that an endotoxin tutor necrosis factor (TNF) acts indirectly by causing the host to release a substance, that mimics the tumour necrotic action of endotoxin and is selectively toxic for malignant cells [64]. Gradually TNF became a rapidly growing prototype family or superfamily with more than 20 ligands and over 29 receptors [65]. There is main two types of membrane-bound receptors- TNFR-1 (expressed by almost all mammalian cell types), TNFR-2 (expressed by mainly immune cells and endothelial cells); that activates through a soluble TNF-a ligand stimulus [64,66]. TNF plays a conflicted role in cancer biology by promoting and suppressing tumour, by promoting angiogenesis along with some other biological activity through various signalling pathways [67,68].
TGF-β: The regulatory cytokine family, transforming growth factor-β (TGF-β) plays multiple roles in embryogenesis, adult angiogenesis and cancer [69]. TGF-β exists as 3 isoforms- TGF-β1, TGF-β2, and TGF-β3 and shows dual roles in both cancer and angiogenesis [69]. There are several specific stromal activators (MMPs, Integrins, ROS, ECM protein, TSP-1 or thrombospondin-1, bone morphogenetic protein 1 or BMP1 etc.) and inhibitors (Proteoglycans, Fibrillin’s, Fibulins, Fibronectin, etc.) that operate latent TGF-β activation and suppression [70]. Although the exact mechanism of TGF-β’s role in angiogenesis is still unclear, but from some preclinical studies it has been observed that tumour angiogenesis stimulates through plasminogen-dependent activation of TGFβRI/ SMAD1/5 [70]. Besides, in the tumour microenvironment, angiogenesis is inhibited when enhanced TGF-β concentration upregulates fibronectin through TGFβRI/ SMAD2/3 signalling pathway [70,71]. Studies in animal models revealed, at early stages of neovascular age-related macular degeneration or nAMD, TGF-β concentration in the aqueous humour decreases and shows protective and antiangiogenic stimulation, while later, in the diseased stage, it shows pro-angiogenic effects, and in human patients, it inhibits tumour development in early stages, whereas in later stages, it supports tumour invasion and metastasis [72-74]. TGF-β1 secreted from radial glia (RG) cells of the brain regulates RG and endothelial cell interaction to form blood vessels resulting in the development of the cerebral cortex [75]. It also regulates melanoma distal metastase, hepatic angiogenesis [76,77]. Leucinerich α-2 glycoprotein (LRG) and Interleukin-37 (IL-37) promote lung fibrosis and angiogenesis, respectively, via TGF-β signalling [78,79]. Besides, TGF-β represses VEGFA-mediated angiogenesis in colon cancer metastasis, breast cancer bone metastase, but stimulate glioblastoma through VEGFR signalling [80,82]. A study on bovine ovaries proved that TGF-β has an inhibitory effect on both the angiogenesis in female reproductive organs and steroidogenesis (formation of steroids) [83]. Studies revealed transforming growth factor-β1 overexpression can cause airway remodelling and lung fibrosis by enhancing collagen Ⅰ and Ⅲ, in mustard lung [84].
PARP-1: PARP (Poly (ADP-ribose) polymerase), recently known as ARTs or ADP-ribosyl transferases, is a protein family including 17 members that have diverse structures, enzymatic activity, subcellular localization and functions [85-87]. Among all, the first discovered and extensively discussed member is PARP-1 or ART-1, a DNA-dependent nuclear enzyme [86,88,89]. Structurally, it consists of 3 domains- 1) DNA-binding region, 2) auto modification region, and 3) catalytic region or the PARP site [90]. In essence, the catalytic region of PARP-1 is associated with DNA damage repair, but in case of severe damage, it induces cell death by NAD+ and ATP depletion [90,91]. It is already proven that PARP and angiogenesis are correlated and PARP-1 inhibitors can suppress the angiogenesis process [92]. The impact of PARP-1 overexpression in cancer angiogenesis is shown in Table 2 [93-96].
MMP: MMPs or matrix metalloproteinases are zinc-dependent catalytic enzyme groups that have a significant contribution in both physiological as well as pathological processes of the human body [97]. There are mainly 24 human MMPs under 6 subfamilies which are associated with the formation of vasculature, destruction of some extracellular matrix (ECM) proteins, like; collagen, etc. and activation of some inflammatory cytokines as well [98]. MMPs are mainly secreted by platelets, fibroblasts, leukocytes, endothelial cells, and vascular smooth muscle as proenzymes [99,100]. Some findings suggest that MMPs promote angiogenesis by activating some signalling pathways and receptors or by enzyme overexpression; besides, it can inhibit angiogenesis related vascular sprouting by converting large proangiogenic molecules into relatively smaller antiangiogenic proteins [101]. Expression of MMPs is a risk factor of cardiovascular disease (CVD), chronic kidney disease (CKD), and Peripheral Vascular Disease (PVD) [99]. MMPs, are also associate with tumour maturity-proliferation-migration and several types of cancer subtypes [101] (Table 3) [102-122]. There are some natural modulators in our body that randomly bind to any MMP in 1:1 ratio and inhibits MMPS, known as tissue inhibitors of metalloproteinases or TIMPs and few synthetic MMP-inhibitors like; metal ions (Cu2+, Mg2+, Mn2+ etc), doxycycline (only MMPi approved by FDA) acts by reducing MMP secretion.
VASH-2: Vasohibin (VASH) consisting of two subfamilies with - VASH-1 and VASH-2, are recently discovered angiogenesis regulator genes that show antipathic effects on tumour angiogenesis; by inhibiting angiogenesis (VASH-1) and by stimulating angiogenesis (VASH-2), although there are 52.5% similarities between full-length human VASH-1 and VASH-2 genes at the amino acid level [123]. VASH-2 generally on exposure to mononuclear cells of bone marrow starts angiogenesis as a chemical stimulator [124]. Studies revealed that VASH-2 can be used as a biomarker in oesophageal squamous cell carcinoma (ESCC) as the plasma concentration level and tumour expression level of VASH-2 were found to increase at a proportional rate [125]. Epigenetic mechanism involving transcriptional start site (TSS) upregulation and activation of histone modifications occurs -354 to -10 region of VASH-2 gene, which probably leads to VASH-2 overexpression following increased angiogenesis in hepatocellular carcinoma (HCC) [126]. VASH-2 expression is related to angiogenesis in human retinal microvascular endothelial cells or HMVEC [127]. VASH-2 promotes tumour angiogenesis by altering gene expression and metastasis by tubulin de-tyrosination of PDAC or Pancreatic Ductal Adenocarcinoma cells [128]. Two growth factors; fibroblast growth factor-2 (FGF-2) and growth/ differentiation factor-15 (GDF-15) overexpression leads to VASH- 2 induced breast cancer cell proliferation [129]. Overexpression of VASH2 indicated as a predictor in oesophageal squamous cell carcinoma (ESCC) and accelerated tumour angiogenesis in some specific types of ovarian cancer by enhancing tumour growth and peritoneal dissemination of tumour cells [130,131].
Experimentally Proven Phytopharmaceutical to Inhibit Vasculature Development
Experimentally proven phytopharmaceuticals inhibiting vasculature development are summarized based on most impacted and cited literature published in the last 15 years; 2005- 2020) as searched from the authenticated databases (Scopus, PubMed, etc), including the keywords like- Pathological Angiogenesis, Cancer Cell Invasion, Metastasis, Cell Migration, Experimentally Proven Phytopharmaceutical, Inhibition of Vasculature Development, Modern Target for Cancer treatment in Table 4. [132-245] A total of 97 plant molecules, studied in this review, including 69 plant species among 40 plant families are summarized in a schematic way. The respective compounds/ extracts are included with their respective sources/ families and the specific protocols/ methodologies used for the experimental proof-of-of-concept studies are tabularized in Table 3 [102-122].
Table 3: MMP subgroups with associated cancer types. [Abbreviations - HCC=Hepatocellular Carcinoma, HNSCC= Head and Neck Squamous Cell Carcinoma, TNBC= Triple Negative Breast Cancer, CNV=Corneal Neovascularization, CLL=Chronic Lymphocytic Leukemia, ESCC=Esophageal Squamous Cell Carcinoma, MCC=Markel Cell Carcinoma, GIT = Gastrointestinal Tract].
Table 4: Experimentally Proven Phytopharmaceutical to Inhibit Vasculature Development. Summarized based on most Impacted and Cited Literature published in last 15 years; 2005- 2020).
Expert Opinion on the Recent Perspectives on Cancer Management
It has been observed that the growth and progression of cancerous tumours beyond a certain size require pathogenic angiogenesis, and therefore angiogenesis inhibition can prove to be an effective strategy in cancer management [246,247]. Typically, the focus on cancer management by angiogenesis inhibition in the recent past has been to develop inhibitors against its stimulant molecules like VEGFR-2, protease inhibitors, etc. [248, 249]. This further with angiogenesis imaging procedures aiding in tumour vasculature characterisation, identification of various biomarkers with the potential to diagnose cancer and even identify patients likely to benefit as well as those with the possibility to develop resistance and/or adverse events from antiangiogenic treatment makes it a promising therapy [250,251]. There is an abundance of experimental phytopharmaceuticals inhibiting angiogenesis however, limited clinical effectivity and high toxicity call for further research in such area [252]. The review presents the physiology of angiogenesis, its stimulants at the molecular level which are basically molecular targets for drug development, mechanisms of angiogenesis, contribution to cancer progression, and a summary of numerous plant compounds/ extracts inhibiting vasculature development along with their families. While the schematic representation of compounds/ extracts having potential anti-vasculature activity together with methods for extraction and development will aid scientists in the timely selection of phytopharmaceuticals for further experimentation, the summarisation of the respective phytochemicals with the plant source/ family would help to trace the origin and provide further scope to identify new plants having potential vasculature development inhibitory activity. Overall, this review will assist in exploring phytopharmaceuticals targeted towards cancer treatment specifically inhibiting vasculature development.
Conclusion
Targeting the tumour vasculature instead of the tumour cells directly is of great interest for tumour management. With regards to this, the review scientifically explained the pathological angiogenesis mechanism responsible for cancer cell invasion and metastasis, and in a similar line, the experimentally proven phytopharmaceuticals having a significant effect inhibiting vasculature development have been represented schematically. Hopefully, this review will facilitate the biomedical scientists in setting up the appropriate research questions around the molecular targets explained here for the management of cancer cell invasion and migration. Therefore, further proof-of-concept validation studies for exploring such phytopharmaceuticals can be possible.
Conflict of Interest
The authors confirm that this article content has no conflicts of interest.
Acknowledgement
The Director and Principal I/C of Guru Nanak Institute of Pharmaceutical Science and Technology are acknowledged. National Institute of Pharmaceutical Education and Research (NIPER), Hajipur, India and “Department of Pharmaceutical, Ministry of Chemical and Fertilizer, Govt. of India” are also acknowledged for providing the M.S. Fellowship to Mr. Antarip Sinha and Ms. Debanjana Das.
References
- Fallah A, Sadeghinia A, Kahroba H, Samadi A, Heidari HR, et al. (2019) Therapeutic targeting of angiogenesis molecular pathways in angiogenesisdependent diseases. Biomed Pharmacothe 110: 775-785.
- Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347): 298-307.
- Ronca R, Benkheil M, Mitola S, Struyf S, Liekens S (2017) Tumor angiogenesis revisited: Regulators and clinical implications. Med Res Rev 37(6): 1231-1274.
- Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21): 1182-1186.
- Nishida N, Yano H, Nishida T, Kamura T, Kojiro M (2006) Angiogenesis in cancer. Vasc Health Risk Manag 2(3): 213-219.
- Liekens S, De Clercq E, Neyts J (2001) Angiogenesis: regulators and clinical applications. BiochemPharmacol 61(3): 253-270.
- Bergers G, Benjamin LE (2003) Tumorigenesis and angiogenic switch. Nat Rev Cancer 3(6): 401-410.
- Kazerounian S, Lawler J (2018) Integration of pro- and anti-angiogenic signals by endothelial cells. J Cell Commun Signal 12(1): 171-179.
- Castañeda-Gill JM, Vishwanatha JK (2016) Antiangiogenic mechanisms and factors in breast cancer treatment. J Carcinog 15: 1.
- Raza A, Franklin MJ, Dudek AZ (2010) Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol 85(8): 593-598.
- Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, et al. (2018) Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J MorpholEmbryol 59(2): 455-467.
- Browne S, Pandit A (2017) Engineered systems for therapeutic angiogenesis. Curr Opin Pharmacol 36: 34-43.
- Marchand M, Monnot C, Muller L, Germain S (2019) Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol 89: 147-156.
- Zimna A, Kurpisz M (2015) Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. Biomed Res Int 2015: 549412.
- Rizzi A, Benagiano V, Ribatti D (2017) Angiogenesis versus arteriogenesis. Rom J MorpholEmbryol 58(1): 15-19.
- Guerra A, Belinha J, Jorge RN (2018) Modelling skin wound healing angiogenesis: A review. J Theor Biol 459: 1-17.
- Lu E, Li C, Wang J, Zhang C (2019) Inflammation and angiogenesis in the corpus luteum. J Obstet Gynaecol Res 45(10): 1967-1974.
- Woad KJ, Robinson RS (2016) Luteal angiogenesis and its control. Theriogenology 86(1): 221-228.
- Chen X, Man GCW, Liu Y, Wu F, Huang J, et al. (2017) Physiological and pathological angiogenesis in the endometrium at the time of embryo implantation. Am J Reprod Immunol 78(2).
- Demir R, Yaba A, Huppertz B (2010) Vasculogenesis and angiogenesis in the endometrium during menstrual cycle and implantation. Acta Histochem 112(3): 203-214.
- Reichard A, Asosingh K (2019) The role of mitochondria in angiogenesis. Mol Biol Rep 46(1): 1393-1400.
- Olfert IM, Baum O, Hellsten Y, Egginton S (2016) Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol 310(3): H326-336.
- Darweesh RS, Ayoub NM, Nazzal S (2019) Gold nanoparticles and angiogenesis: molecular mechanisms and biomedical applications. Int J Nanomedicine 14: 7643-7663.
- Rizov M, Andreeva P, Dimova I (2017) Molecular regulation and role of angiogenesis in reproduction. Taiwan J Obstet Gynecol 56(2): 127-132.
- Elmasry K, Ibrahim AS, Abdulmoneim S, Al-Shabrawey M (2019) Bioactive lipids and pathological retinal angiogenesis. Br J Pharmacol 176(1): 93-109.
- Li Y, Zhou Y (2019) Interleukin-17: The Role of Pathological Angiogenesis in Ocular Neovascular Diseases. Tohoku J Exp Med 247(2): 87-98.
- Capitão M, Soares R (2016) Angiogenesis and Inflammation Crosstalk in Diabetic Retinopathy. J Cell Biochem 117(11): 2443-2453.
- Adini A, Adini I, Chi ZL, Derda R, Birsner AE (2017) A novel strategy to enhance angiogenesis in vivo is using the small VEGF-binding peptide PR1P. Angiogenesis 20(3): 399-408.
- Johnson T, Zhao L, Manuel G, Taylor H, Liu D (2019) Approaches to therapeutic angiogenesis for ischemic heart disease. J Mol Med (Berl) 97(2): 141-151.
- Naito H, Iba T, Takakura N (2020) Mechanisms of new blood vessel formation and proliferative heterogeneity of endothelial cells. Int Immunol 32(5): 295-305.
- Castro PR, Barbosa AS, Pereira JM, Ranfley H, Felipetto M, et al. (2018) Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes. Biomed Res Int 2018: 6740408.
- De Saedeleer CJ, Copetti T, Porporato PE, Verrax J, Feron O, et al. (2012) Lactate activates HIF-1 in oxidative but not in Warburg-phenotype human tumor cells. PLoS One 7(10): e46571.
- Vallée A, Guillevin R, Vallée JN (2018) Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/β-catenin pathway in gliomas. Rev Neurosci 29(1): 71-91.
- Moccia F, Negri S, Shekha M, Faris P, Guerra G (2019) Endothelial Ca2+ Signaling, Angiogenesis and Vasculogenesis: just What It Takes to Make a Blood Vessel. Int J Mol Sci 20(16): 3962.
- Simons KH, Aref Z, Peters HAB, Welten SP, Nossent AY, et al. (2018) The role of CD27-CD70-mediated T cell co-stimulation in vasculogenesis, arteriogenesis, and angiogenesis. Int J Cardiol 260: 184-190.
- Ribatti D, Crivellato E (2012) Sprouting angiogenesis, a reappraisal. Dev Biol 372(2): 157-165.
- Oliveira de Oliveira LB, FaccinBampi V, Ferreira Gomes C, Braga da Silva JL, EncarnaçãoFiala Rechsteiner SM (2014) Morphological characterization of sprouting and intussusceptive angiogenesis by SEM in oral squamous cell carcinoma. Scanning 36(3): 293-300.
- Saravanan S, Vimalraj S, Pavani K, Nikarika R, Sumantran VN (2020) Intussusceptive angiogenesis as a key therapeutic target for cancer therapy. Life Sci 252: 117670.
- Duran CL, Howell DW, Dave JM, Smith RL, Torrie ME, et al. (2017) Molecular Regulation of Sprouting Angiogenesis. Compr Physiol 8(1): 153-235.
- Sacilotto N, Chouliaras KM, Nikitenko LL, Lu YW, Fritzsche M, et al. (2016) MEF2 transcription factors are key regulators of sprouting angiogenesis. Genes Dev 30(20): 2297-2309.
- Díaz-Flores L, Gutiérrez R, Gayoso S, García MP, González-Gómez M, et al. (2020) Intussusceptive angiogenesis and its counterpart intussusceptive lymphangiogenesis. HistolHistopathol 35(10): 1083-1103.
- Ribatti D, Djonov V (2012) Intussusceptive microvascular growth in tumors. Cancer Lett 316(2): 126-131.
- Burri PH, Hlushchuk R, Djonov V (2004) Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn 231(3): 474-488.
- Zanotelli MR, Reinhart-King CA (2018) Mechanical Forces in Tumor Angiogenesis. Adv Exp Med Biol 1092: 91-112.
- Prior BM, Yang HT, Terjung RL (2004) What makes vessels grow with exercise training? J ApplPhysiol (1985) 97(3): 1119-1128.
- Frezzetti D, Gallo M, Maiello MR, D'Alessio A, Esposito C, (2017) VEGF as a potential target in lung cancer. Expert OpinTher Targets 21(10): 959-966.
- Matsumoto K, Ema M (2014) Roles of VEGF-A signalling in development, regeneration, and tumours. J Biochem 156(1): 1-10.
- Lee SH, Jeong D, Han YS, Baek MJ (2015) Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 89(1): 1-8.
- Theis V, Theiss C (2018) VEGF - A Stimulus for Neuronal Development and Regeneration in the CNS and PNS. Curr Protein Pept Sci 19(6): 589-597.
- Apte RS, Chen DS, Ferrara N (2019) VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176(6): 1248-1264.
- Buijs N, Oosterink JE, Jessup M, Schierbeek H, Stolz DB, et al. (2017) A new key player in VEGF-dependent angiogenesis in human hepatocellular carcinoma: dimethylarginine dimethylaminohydrolase 1. Angiogenesis 20(4): 557-565.
- Chen Y, Zhang L, Liu WX, Wang K (2018) VEGF and SEMA4D have synergistic effects on the promotion of angiogenesis in epithelial ovarian cancer. Cell Mol Biol Lett 23: 2.
- Hou P, Li H, Yong H, Chen F, Chu S, et al. (2019) PinX1 represses renal cancer angiogenesis via the mir-125a-3p/VEGF signaling pathway. Angiogenesis 22(4): 507-519.
- Adini A, Adini I, Ghosh K, Benny O, Pravda, et al. (2013) The stem cell marker prominin-1/CD133 interacts with vascular endothelial growth factor and potentiates its action. Angiogenesis 16(2): 405-416.
- Chiu DK, Zhang MS, Tse AP, Wong CC (2019) Assessment of Stabilization and Activity of the HIFs Important for Hypoxia-Induced Signalling in Cancer Cells. Methods Mol Biol 1928: 77-99.
- Wigerup C, Påhlman S, Bexell D (2016) Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. PharmacolTher 164: 152-169.
- Loboda A, Jozkowicz A, Dulak J (2012) HIF-1 versus HIF-2--is one more important than the other? VasculPharmacol 56(5-6): 245-251.
- Semenza GL (2014) Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76: 39-56.
- Rankin EB, Giaccia AJ (2008) The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ 15(4): 678-685.
- Rey S, Semenza GL (2010) Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86(2): 236-242.
- Toberer F, Haenssle HA, Heinzel-Gutenbrunner M, Enk A, Hartschuh W, et al. (2021) Metabolic reprogramming and angiogenesis in primary cutaneous Merkel cell carcinoma: expression of hypoxia-inducible factor-1α and its central downstream factors. J Eur Acad Dermatol Venereol 35(1): 88-94.
- Bigatto V, De Bacco F, Casanova E, Reato G, Lanzetti L, et al. (2015) TNF-α promotes invasive growth through the MET signaling pathway. Mol Oncol 9(2): 377-388.
- Moreira-Tabaka H, Peluso J, Vonesch JL, Hentsch D, Kessler P, et al. (2012) Unlike for human monocytes, after LPS activation, the release of TNF-α by THP-1 cells is produced by TACE catalytically different from constitutive TACE. PLoSOne 7(3): e34184.
- Brenner D, Blaser H, Mak TW (2015) Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol 15(6): 362-374.
- Grewal IS (2009) Overview of TNF superfamily: a chest full of potential therapeutic targets. Adv Exp Med Biol 647: 1-7.
- Richter C, Messerschmidt S, Holeiter G, Tepperink J, Osswald S, et al. (2012) The tumor necrosis factor receptor stalk regions define responsiveness to soluble versus membrane-bound ligand. Mol Cell Biol 32(13): 2515-2529.
- Bertazza L, Mocellin S (2010) The dual role of tumor necrosis factor (TNF) in cancer biology. Curr Med Chem 17(29): 3337-3352.
- Wang X, Lin Y (2008) Tumor necrosis factor and cancer buddies or fo? Acta Pharmacol Sin 29(11): 1275-1288.
- Maring JA, van Meeteren LA, Goumans MJ, Ten Dijke P (2016) Interrogating TGF-β Function and Regulation in Endothelial Cells. Methods Mol Biol 1344: 193-203.
- Costanza B, Umelo IA, Bellier J, Castronovo V, Turtoi A (2017) Stromal Modulators of TGF-β in Cancer. J Clin Med 6(1): 7.
- Zonneville J, Safina A, Truskinovsky AM, Arteaga CL, Bakin AV (2018) TGF-β signaling promotes tumor vasculature by enhancing the pericyte-endothelium association. BMC Cancer 18(1): 670.
- Tosi GM, Orlandini M, Galvagni F (2018) The Controversial Role of TGF-β in Neovascular Age-Related Macular Degeneration Pathogenesis. Int J Mol Sci 19(11): 3363.
- Tosi GM, Neri G, Caldi E, Fusco F, Bacci T, et al. (2018) TGF-β concentrations and activity are downregulated in the aqueous humor of patients with neovascular age-related macular degeneration. Sci Rep 8(1): 8053.
- Syed V (2016) TGF-β Signaling in Cancer. J Cell Biochem 117(6): 1279-1287.
- Siqueira M, Francis D, Gisbert D, Gomes FCA, Stipursky J (2018) Radial Glia Cells Control Angiogenesis in the Developing Cerebral Cortex Through TGF-β1 Signaling. Mol Neurobiol 55(5): 3660-3675.
- Lauden L, Siewiera J, Boukouaci W, Ramgolam K, Mourah S, et al. (2014) TGF-β-induced (TGFBI) protein in melanoma: a signature of high metastatic potential. J Invest Dermatol 134(6): 1675-1685.
- Jin X, Aimaiti Y, Chen Z, Wang W, Li D (2018) Hepatic stellate cells promote angiogenesis via the TGF-β1-Jagged1/VEGFA axis. Exp Cell Res 373(1-2): 34-43.
- Honda H, Fujimoto M, Serada S, Urushima H, Mishima T, et al. (2017) Leucine-rich α-2 glycoprotein promotes lung fibrosis by modulating TGF-β signaling in fibroblasts. Physiol Rep 5(24): e13556.
- Zhao M, Hu Y, Jin J, Yu Y, Zhang S, et al. (2017) Interleukin 37 promotes angiogenesis through TGF-β Sci Rep 7(1): 6113.
- Geng L, Chaudhuri A, Talmon G, Wisecarver JL, Wang J (2013) TGF-Beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS One 8(3): e59918.
- Chiechi A, Waning DL, Stayrook KR, Buijs JT, Guise TA, et al. (2013) Role of TGF-β in breast cancer bone metastases. Adv BiosciBiotechnol 4(10C): 15-30.
- Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, et al. (2015) Modulation of cerebral endothelial cell function by TGF-β in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget 6(26): 22480-22495.
- Mattar D, Samir M, Laird M, Knight PG (2020) Modulatory effects of TGF-β1 and BMP6 on the cal angiogenesis and steroidogenesis in the bovine ovary. Reproduction 159(4): 397-408.
- Ghane Zadeh F, Mirzamani MS, Halabiyan R, Mahmoodzadeh Hosseini H, Imani Fooladi AA, et al. (2015) The effects of sulfur mustard on the expression of TGF-βs variants in lung epithelial cell lines. J Recept Signal Transduct Res 35(4): 284-288.
- Hottiger MO, Hassa PO, Lüscher B, Schüler H, Koch-Nolte F (2010) Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci. Apr 35(4): 208-219.
- Jubin T, Kadam A, Jariwala M, Bhatt S, Sutariya S, et al. (2016) The PARP family: insights into functional aspects of poly (ADP-ribose) polymerase-1 in cell growth and survival. Cell Prolif 49(4): 421-437.
- Vyas S, Matic I, Uchima L, Rood J, Zaja R, et al. (2014) Family-wide analysis of poly (ADP-ribose) polymerase activity. Nat Commun 5: 4426.
- Gupte R, Liu Z, Kraus WL (2017) PARPs and ADP-ribosylation: recent advances linking molecular functions to biological outcomes. Genes Dev 31(2): 101-126.
- Hassler M, Ladurner AG (2012) Towards a structural understanding of PARP1 activation and related signalling ADP-ribosyltransferases. CurrOpin Struct Biol 22(6): 721-729.
- Rodríguez MI, Majuelos-Melguizo J, Martí Martín-Consuegra JM, Ruiz de Almodóvar M, López-Rivas A, et al. (2015) Deciphering the insights of poly (ADP-ribosylation) in tumor progression. Med Res Rev 35(4): 678-697.
- Wei W, Li Y, Lv S, Zhang C, Tian Y (2016) PARP-1 may be involved in angiogenesis in epithelial ovarian cancer. Oncol Lett 12(6): 4561-4567.
- Tentori L, Lacal PM, Muzi A, Dorio AS, Leonetti C, et al. (2007) Poly (ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer 43(14): 2124-2133.
- Li Q, Li M, Wang YL, Fauzee NJ, Yang Y, et al. (2012) RNA interference of PARG could inhibit the metastatic potency of colon carcinoma cells via PI3-kinase/Akt pathway. Cell PhysiolBiochem 29(3-4): 361-372.
- Martínez-Bosch N, Iglesias M, Munné-Collado J, Martínez-Cáceres C, Moreno M, et al. (2014) Parp-1 genetic ablation in Ela-myc mice unveils novel roles for Parp-1 in pancreatic cancer. J Pathol 234(2): 214-227.
- Rodríguez MI, Peralta-Leal A, O'Valle F, Rodriguez-Vargas JM, Gonzalez-Flores A, et al. (2013) PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet 9(6): e1003531.
- Rojo F, García-Parra J, Zazo S, Tusquets I, Ferrer-Lozano J, et al. (2012) Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann Oncol 23(5): 1156-1164.
- Cui N, Hu M, Khalil RA (2017) Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog Mol Biol Transl Sci 147: 1-73.
- Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, et al. (2019) Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol Hematol 137: 57-83.
- Provenzano M, Andreucci M, Garofalo C, Faga T, Michael A, et al. (2020) The Association of Matrix Metalloproteinases with Chronic Kidney Disease and Peripheral Vascular Disease: ¿A Light at the End of the Tunnel? Biomolecules 10(1): 154.
- Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, et al. (2019) Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol 9: 1370.
- Roy R, Morad G, Jedinak A, Moses MA (2020) Metalloproteinases and their roles in human cancer. Anat Rec (Hoboken) 303(6): 1557-1572.
- Zhang J, Wang S, He Y, Yao B, Zhang Y (2020) Regulation of matrix metalloproteinases 2 and 9 in corneal neovascularization. Chem Biol Drug Des 95(5): 485-492.
- Wang X, Khalil RA (2018) Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv Pharmacol 81: 241-330.
- Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, et al. (2019) The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal Cell Pathol (Amst) 2019: 9423907.
- Böckelman C, Beilmann-Lehtonen I, Kaprio T, Koskensalo S, Tervahartiala T, et al. (2018) Serum MMP-8 and TIMP-1 predicted prognosis in colorectal cancer. BMC Cancer 18(1): 679.
- Laitinen A, Hagström J, Mustonen H, Kokkola A, Tervahartiala T, et al. (2018) Serum MMP-8 and TIMP-1 as prognostic biomarkers in gastric cancer. Tumour Biol 40(9): 1010428318799266.
- Shen TC, Hsia TC, Chao CY, Chen WC, Chen CY, et al. (2017) The Contribution of MMP-8 Promoter Polymorphisms in Lung Cancer. Anticancer Res 37(7): 3563-3567.
- Zhang H, Yang Q, Lian X, Jiang P, Cui J (2019) Hypoxia-Inducible Factor-1α (HIF-1α) Promotes Hypoxia-Induced Invasion and Metastasis in Ovarian Cancer by Targeting Matrix Metallopeptidase 13 (MMP13). Med Sci Monit 25: 7202-7208.
- Ma Y, Cang S, Li G, Su Y, Zhang H, et al. (2019) Integrated analysis of transcriptome data revealed MMP3 and MMP13 as critical genes in anaplastic thyroid cancer progression. J Cell Physiol 234(12): 22260-22271.
- Li Y, Song T, Chen Z, Wang Y, Zhang J, et al. (2019) Pancreatic Stellate Cells Activation and Matrix Metallopeptidase 2 Expression Correlate with Lymph Node Metastasis in Pancreatic Carcinoma. Am J Med Sci 357(1): 16-22.
- Radisky ES, Raeeszadeh-Sarmazdeh M, Radisky DC (2017) Therapeutic Potential of Matrix Metalloproteinase Inhibition in Breast Cancer. J Cell Biochem 118(11): 3531-3548.
- Aguilera-Montilla N, Bailón E, Ugarte-Berzal E, Uceda-Castro R, Prieto-Solano M, et al. (2019) Matrix metalloproteinase-9 induces a pro-angiogenic profile in chronic lymphocytic leukemia cells. BiochemBiophys Res Commun 520(1): 198-204.
- Li H, Yang F, Chai L, Zhang L, Li S, et al. (2019) CCAAT/Enhancer Binding Protein β-Mediated MMP3 Upregulation Promotes Esophageal Squamous Cell Cancer Invasion InVitro and Is Associated with Metastasis in Human Patients. Genet Test Mol Biomarkers 23(5): 304-309.
- Guo JG, Guo CC, He ZQ, Cai XY, Mou YG (2018) High MMP-26 expression in glioma is correlated with poor clinical outcome of patients. Oncol Lett 16(2): 2237-2242.
- Truong A, Yip C, Paye A, Blacher S, Munaut C, et al. (2016) Dynamics of internalization and recycling of the prometastatic membrane type 4 matrix metalloproteinase (MT4-MMP) in breast cancer cells. FEBS J 283(4): 704-722.
- Moogk D, da Silva IP, Ma MW, Friedman EB, de Miera EV, et al. (2014) Melanoma expression of matrix metalloproteinase-23 is associated with blunted tumor immunity and poor responses to immunotherapy. J Transl Med 12: 342.
- Sugimoto W, Itoh K, Hirata H, Abe Y, Torii T, et al. (2020) MMP24 as a Target of YAP is a Potential Prognostic Factor in Cancer Patients. Bioengineering (Basel) 7(1): 18.
- El-Ashmawy NE, Khedr NF, Mansour MG, Al-Ashmawy GM (2020) TNM staging for GIT cancers is correlated with the level of MMPs and TGF-β Clin Exp Med 20(4): 545-555.
- Wang S, Jia J, Liu D, Wang M, Wang Z, et al. (2019) Matrix Metalloproteinase Expressions Play Important role in Prediction of Ovarian Cancer Outcome. Sci Rep 9(1): 11677.
- Zhao Z, Yan L, Li S, Sun H, Zhou Y, et al. (2014) Increased MMP-21 expression in esophageal squamous cell carcinoma is associated with progression and prognosis. Med Oncol 31(8): 91.
- Suomela S, Koljonen V, Skoog T, Kukko H, Böhling T, et al. (2009) Expression of MMP-10, MMP-21, MMP-26, and MMP-28 in Merkel cell carcinoma. Virchows Arch 455(6): 495-503.
- Murugan AK, Yang C, Xing M (2013) Mutational analysis of the GNA11, MMP27, FGD1, TRRAP and GRM3 genes in thyroid cancer. Oncol Lett 6(2): 437-441.
- Sato Y (2013) The vasohibin family: a novel family for angiogenesis regulation. J Biochem 153(1): 5-11.
- Sato Y (2012) The vasohibin family: Novel regulators of angiogenesis. VasculPharmacol 56(5-6): 262-266.
- Yamamoto M, Ozawa S, Ninomiya Y, Koyanagi K, Oguma J, et al. (2020) Plasma vasohibin-1 and vasohibin-2 are useful biomarkers in patients with esophageal squamous cell carcinoma. Esophagus 17(3): 289-297.
- Xue X, Gao W, Sun B, Xu Y, Han B, et al. (2013) Vasohibin 2 is transcriptionally activated and promotes angiogenesis in hepatocellular carcinoma. Oncogene 32(13): 1724-1734.
- Hu XN, Ni Y, Luan J, Ding YZ (2020) A review on vasohibin and ocular neovascularization. Int J Ophthalmol 13(6): 1004-1008.
- Iida-Norita R, Kawamura M, Suzuki Y, Hamada S, Masamune A, et al. (2019) Vasohibin-2 plays an essential role in metastasis of pancreatic ductal adenocarcinoma. Cancer Sci 110(7): 2296-2308.
- Tu M, Liu X, Han B, Ge Q, Li Z, et al. (2014) Vasohibin-2 promotes proliferation inhuman breast cancer cells via upregulation of fibroblast growth factor-2 and growth/differentiation factor-15 expression. Mol Med Rep 10(2): 663-669.
- Ninomiya Y, Ozawa S, Oguma J, Kazuno A, Nitta M, et al. (2018) Expression of vasohibin-1 and -2 predicts poor prognosis among patients with squamous cell carcinoma of the esophagus. Oncol Lett 16(4): 5265-5274.
- Takahashi Y, Koyanagi T, Suzuki Y, Saga Y, Kanomata N, et al. (2012) Vasohibin-2 expressed inhuman serous ovarian adenocarcinoma accelerates tumor growth by promoting angiogenesis. Mol Cancer Res 10(9): 1135-1146.
- Chung TW, Kim EY, Choi HJ, Han CW, Jang SB, et al. (2019) 6'-Sialylgalactose inhibits vascular endothelial growth factor receptor 2-mediated angiogenesis. Exp Mol Med 51(10): 1-13.
- Li J, Wu Y, Wang D, Zou L, Fu C, et al. (2019) Oridonin synergistically enhances the anti-tumor efficacy of doxorubicin against aggressive breast cancer via pro-apoptotic and anti-angiogenic effects. Pharmacol Res 146: 104313.
- Dong Y, Zhang T, Li J, Deng H, Song Y, et al. (2014) Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the Notch signaling. PLoS One 9(12): e113830.
- Razak NA, Abu N, Ho WY, Zamberi NR, Tan SW, et al. (2019) Cytotoxicity of eupatorin in MCF-7 and MDA-MB-231 human breast cancer cells via cell cycle arrest, anti-angiogenesis and induction of apoptosis. Sci Rep 9(1): 1514.
- Lin W, Tu H, Zhu Y, Guan Y, Liu H, et al. (2019) Curcumolide, a unique sesquiterpenoid from Curcuma wenyujin displays anti-angiogenic activity and attenuates ischemia-induced retinal neovascularization. Phytomedicine 64: 152923.
- Othman Zulhabri, Khalep Hamimi, Zainal Abidin Azrina, Hassan Halijah, Fattepur Santosh (2019) The Anti-Angiogenic Properties of L (Mengkudu) Leaves Using Chicken Chorioallantoic Membrane (CAM) Assay. Pharmacognosy Journal 11(1): 12-15.
- Carrillo P, Martínez-Poveda B, Cheng-Sánchez I, Guerra J, Tobia C, et al. (2019) Exploring the Antiangiogenic Potential of SolomonamideA Bioactive Precursors: In Vitro and in Vivo Evidence of the Inhibitory Activity of Solo F-OH During Angiogenesis. Mar Drugs 17(4): 228.
- Meng N, Mu X, Lv X, Wang L, Li N, et al. (2019) Autophagy represses fascaplysin-induced apoptosis and angiogenesis inhibition via ROS and p8 in vascular endothelia cells. Biomed Pharmacother 114: 108866.
- Jin W, Wu W, Tang H, Wei B, Wang H, et al. (2019) Structure Analysis and Anti-Tumor and Anti-Angiogenic Activities of SulfatedGalactofucan Extracted from Sargassum thunbergii. Mar Drugs 17(1): 52.
- Carrillo P, Martínez-Poveda B, Medina MÁ, Quesada AR (2019) The strigolactoneanalog GR-24 inhibits angiogenesis in vivo and in vitro by a mechanism involving cytoskeletal reorganization and VEGFR2 signalling. BiochemPharmacol 168: 366-383.
- Cho HD, Kim JH, Park JK, Hong SM, Kim DH, et al. (2019) Kochia scoparia seed extract suppresses VEGF-induced angiogenesis via modulating VEGF receptor 2 and PI3K/AKT/mTOR pathways. Pharm Biol 57(1): 684-693.
- Kamble S, Gacche R (2019) Evaluation of Anti-breast cancer, Antiangiogenic and Antioxidant Properties of Selected Medicinal Plants. European Journal of Integrative Medicine 25: 13-19.
- Sun D, Zhang F, Qian J, Shen W, Fan H, et al. (2018) 4'-hydroxywogonin inhibits colorectal cancer angiogenesis by disrupting PI3K/AKT signaling. Chem Biol Interact 296: 26-33.
- Hu H, Huang G, Wang H, Li X, Wang X, et al. (2018) Inhibition effect of triptolide on human epithelial ovarian cancer via adjusting cellular immunity and angiogenesis. Oncol Rep 39(3): 1191-1196.
- He MF, Liu L, Ge W, Shaw PC, Jiang R, et al. (2009) Antiangiogenic activity of Tripterygium wilfordii and its terpenoids. J Ethnopharmacol 121(1): 61-68.
- Sonowal H, Shukla K, Kota S, Saxena A, Ramana KV (2018) Vialinin A, an Edible Mushroom-Derived p-Terphenyl Antioxidant, Prevents VEGF-Induced Neovascularization In Vitro and In Vivo. Oxid Med Cell Longev 2018: 1052102.
- Piao XM, Gao F, Zhu JX, Wang LJ, Zhao X, et al. (2018) Cucurbitacin B inhibits tumor angiogenesis by triggering the mitochondrial signaling pathway in endothelial cells. Int J Mol Med 42(2): 1018-1025.
- Bala PK, Mukherjee FC, Braga MG, Matsabisa (2018) Comparative inhibition of MCF-7 breast cancer cell growth, invasion and angiogenesis by Cannabis sativa L. sourced from sixteen different geographic locations. South African Journal of Botany 119: 154-162.
- Ramer R, Fischer S, Haustein M, Manda K, Hinz B (2014) Cannabinoids inhibit angiogenic capacities of endothelial cells via release of tissue inhibitor of matrix metalloproteinases-1 from lung cancer cells. BiochemPharmacol 91(2): 202-216.
- Shen M, Zhou XZ, Ye L, Yuan Q, Shi C, et al. (2018) Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2-mediated STAT3/PI3K/Akt signaling pathway. Int J Mol Med 42(2): 769-778.
- Yu Y, Yu J, Pei CG, Li YY, Tu P, et al. (2015) Xanthatin, a novel potent inhibitor of VEGFR2 signaling, inhibits angiogenesis and tumor growth in breast cancer cells. Int J Clin Exp Pathol 8(9): 10355-10364.
- Romero M, Zanuy M, Rosell E, Cascante M, Piulats J, et al. (2015) Optimization of xanthatin extraction from Xanthium spinosum L. and its cytotoxic, anti-angiogenesis and antiviral properties. Eur J Med Chem 90: 491-496.
- Mi C, Ma J, Wang KS, Zuo HX, Wang Z, et al. (2017) Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J Ethnopharmacol 203: 27-38.
- Yu S, Oh J, Li F, Kwon Y, Cho H, et al. (2017) New Scaffold for Angiogenesis Inhibitors Discovered by Targeted Chemical Transformations of Wondonin Natural Products. ACS Med Chem Lett 8(10): 1066-1071.
- Huang Y, Miao Z, Hu Y, Yuan Y, Zhou Y, et al. (2017) Baicalein reduces angiogenesis in the inflammatory microenvironment via inhibiting the expression of AP-1. Oncotarget 8(1): 883-899.
- Joshua M, Okere C, Sylvester O, Yahaya M, Omale Precious, et al. (2017) Disruption of Angiogenesis by Anthocyanin-Rich Extracts of Hibiscus sabdariffa. Int J Sci Eng Res 8(2): 299-307.
- Tae N, Hung TM, Kim O, Kim N, Lee S,et al. (2017) A cassaine diterpene alkaloid, 3β-acetyl-nor-erythrophlamide, suppresses VEGF-induced angiogenesis and tumor growth via inhibiting eNOS activation. Oncotarget 8(54): 92346-92358.
- Chen Y, Tian JL, Wu JS, Sun TM, Zhou LN, et al. (2017) Biotransfomation of cyperenoic acid by Cunninghamella elegans AS 3.2028 and the potent anti-angiogenic activities of its metabolites. Fitoterapia 118: 32-37.
- Lee S, Park JY, Lee D, Seok S, Kwon YJ, et al. (2017) Chemical constituents from the rare mushroom Calvatia nipponica inhibit the promotion of angiogenesis in HUVECs. Bioorg Med Chem Lett 27(17): 4122-4127.
- Dongare S, Gupta SK, Mathur R, Saxena R, Mathur S, et al. (2016) Zingiber officinale attenuates retinal microvascular changes in diabetic rats via anti-inflammatory and antiangiogenic mechanisms. Mol Vis 22: 599-609.
- Sousa M, Machado V, Costa R, Figueira ME, Sepodes B, et al. (2016) Red Raspberry Phenols Inhibit Angiogenesis: A Morphological and Subcellular Analysis Upon Human Endothelial Cells. J Cell Biochem 117(7): 1604-1612.
- Tabana YM, Hassan LE, Ahamed MB, Dahham SS, Iqbal MA, et al. (2016) Scopoletin, an active principle of tree tobacco (Nicotiana glauca) inhibits human tumor vascularization in xenograft models and modulates ERK1, VEGF-A, and FGF-2 in computer model. Microvasc Res 107: 17-33.
- Pan R, Gao X, Lu D, Xu X, Xia Y, et al. (2011) Prevention of FGF-2-induced angiogenesis by scopoletin, a coumarin compound isolated from ErycibeobtusifoliaBenth, and its mechanism of action. Int Immunopharmacol 11(12): 2007-2016.
- Mena HA, Carestia A, Scotti L, Parborell F, Schattner M, et al. (2016) Extracellular histones reduce survival and angiogenic responses of late outgrowth progenitor and mature endothelial cells. J Thromb Haemost 14(2): 397-410.
- Valli M, Altei W, Santos R, Jr E, Dessoy M, et al. (2016) Synthetic Analogue of the Natural Product Piperlongumine as a Potent Inhibitor of Breast Cancer Cell Line Migration. Journal of the Brazilian Chemical Society 28(3).
- García-Vilas JA, Pino-Ángeles A, Martínez-Poveda B, Quesada AR, Medina MÁ (2017) The noni anthraquinone damnacanthal is a multi-kinase inhibitor with potent anti-angiogenic effects. Cancer Lett 385: 1-11.
- Cao W, Hu C, Wu L, Xu L, Jiang W (2016) Rosmarinic acid inhibits inflammation and angiogenesis of hepatocellular carcinoma by suppression of NF-κBsignaling in H22 tumor-bearing mice. J Pharmacol Sci 132(2): 131-137.
- Kim JH, Lee BJ, Kim JH, Yu YS, Kim MY, et al. (2009) Rosmarinic acid suppresses retinal neovascularization via cell cycle arrest with increase of p21(WAF1) expression. Eur J Pharmacol 615(1-3): 150-154.
- Huang SS, Zheng RL (2006) Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett 239(2): 271-280.
- Sumer Bolu B, ManavogluGecici E, Sanyal R (2016) Combretastatin A-4 Conjugated Antiangiogenic Micellar Drug Delivery Systems Using Dendron-Polymer Conjugates. Mol Pharm 13(5): 1482-1490.
- Li Q, Wang X, Dai T, Liu C, Li T, et al. (2016) Proanthocyanidins, Isolated from Choerospondiasaxillaris Fruit Peels, Exhibit Potent Antioxidant Activities in Vitro and a Novel Anti-angiogenic Property in Vitro and in Vivo. J Agric Food Chem 64(18): 3546-356.
- Huang S, Yang N, Liu Y, Hu L, Zhao J, et al. (2012) Grape seed proanthocyanidins inhibit angiogenesis via the downregulation of both vascular endothelial growth factor and angiopoietin signaling. Nutr Res 32(7): 530-536.
- Huang W, Liang Y, Wang J, Li G, Wang G, et al. (2016) Anti-angiogenic activity and mechanism of kaurane diterpenoids from Wedelia chinensis. Phytomedicine 23(3): 283-292.
- Bhutia SK, Behera B, Nandini Das D, Mukhopadhyay S, Sinha N, et al. (2016) Abrus agglutinin is a potent anti-proliferative and anti-angiogenic agent in human breast cancer. Int J Cancer 139(2): 457-466.
- Kuo ZK, Lin MW, Lu IH, Yao HJ, Wu HC, et al. (2016) Antiangiogenic and antihepatocellular carcinoma activities of the Juniperus chinensis extract. BMC Complement Altern Med 16: 277.
- Lee SR, Park JY, Yu JS, Lee SO, Ryu JY, et al. (2016) Odisolane, a Novel Oxolane Derivative, and Antiangiogenic Constituents from the Fruits of Mulberry (Morus alba L.). J Agric Food Chem 64(19): 3804-3809.
- Jin S, Yun HJ, Jeong HY, Oh YN, Park HJ, et al. (2015) Widdrol, a sesquiterpene isolated from Juniperus chinensis, inhibits angiogenesis by targeting vascular endothelial growth factor receptor 2 signaling. Oncol Rep 34(3): 1178-1184.
- Li F, Bai Y, Zhao M, Huang L, Li S, et al. (2015) Quercetin inhibits vascular endothelial growth factor-induced choroidal and retinal angiogenesis in vitro. Ophthalmic Res 53(3): 109-116.
- Ravishankar D, Watson KA, Boateng SY, Green RJ, Greco F, et al. (2015) Exploring quercetin and luteolin derivatives as antiangiogenic agents. Eur J Med Chem 97: 259-274.
- Zhao D, Qin C, Fan X, Li Y, Gu B (2014) Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur J Pharmacol 723: 360-367.
- Chen Y, Li XX, Xing NZ, Cao XG (2008) Quercetin inhibits choroidal and retinal angiogenesis in vitro. Graefes Arch Clin Exp Ophthalmol 246(3): 373-378.
- Tan WF, Lin LP, Li MH, Zhang YX, Tong YG, et al. (2003) Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur J Pharmacol 459(2-3): 255-262.
- Pierpaoli E, Damiani E, Orlando F, Lucarini G, Bartozzi B, et al. (2015) Antiangiogenic and antitumor activities of berberine derivative NAX014 compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis 36(10): 1169-1179.
- Chu SC, Yu CC, Hsu LS, Chen KS, Su MY, et al. (2014) Berberine reverses epithelial-to-mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol Pharmacol 86(6): 609-623.
- Hamsa TP, Kuttan G (2012) Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem Toxicol 35(1): 57-70.
- Jie S, Li H, Tian Y, Guo D, Zhu J, et al. (2011) Berberine inhibits angiogenic potential of Hep G2 cell line through VEGF down-regulation in vitro. J Gastroenterol Hepatol 26(1): 179-185.
- Gao JL, Shi JM, Lee SM, Zhang QW, Wang YT (2009) Angiogenic pathway inhibition of Corydalis yanhusuo and berberine in human umbilical vein endothelial cells. Oncol Res 17(11-12): 519-526.
- Qi J, Xia G, Huang CR, Wang JX, Zhang J (2015) JSI-124 (Cucurbitacin I) inhibits tumor angiogenesis of human breast cancer through reduction of STAT3 phosphorylation. Am J Chin Med 43(2): 337-347.
- Ambasta RK, Jha SK, Kumar D, Sharma R, Jha NK, et al. (2015) Comparative study of anti-angiogenic activities of luteolin, lectin and lupeol biomolecules. J Transl Med 13: 307.
- Bagli E, Stefaniotou M, Morbidelli L, Ziche M, Psillas K, et al. (2004) Luteolin inhibits vascular endothelial growth factor-induced angiogenesis; inhibition of endothelial cell survival and proliferation by targeting phosphatidylinositol 3'-kinase activity. Cancer Res 64(21): 7936-7946.
- Wang JJ, Chung HY, Zhang YB, Li GQ, Li YL, et al. (2016) Diterpenoids from the roots of Croton crassifolius and their anti-angiogenic activity. Phytochemistry 122: 270-275.
- Adaramoye O, Erguen B, Oyebode O, Nitzsche B, Höpfner M, et al. (2015) Antioxidant, antiangiogenic and antiproliferative activities of root methanol extract of Calliandraportoricensis in human prostate cancer cells. J Integr Med 13(3): 185-193.
- Huang W, Wang J, Liang Y, Ge W, Wang G, et al. (2015) Potent anti-angiogenic component in Croton crassifolius and its mechanism of action. J Ethnopharmacol 175: 185-191.
- Hung TM, Cuong TD, Kim JA, Tae N, Lee JH, et al. (2014) Cassaine diterpene alkaloids from Erythrophleumfordii and their anti-angiogenic effect. Bioorg Med Chem Lett 24(1): 168-172.
- Kim HJ, Kim JK (2015) Antiangiogenic effects of cucurbitacin-I. Arch Pharm Res 38(2): 290-298.
- Karthik G, Angappan M, Vijay A, Natarajapillai S (2014) Syringic acid exerts antiangiogenic activity by downregulation of VEGF in zebrafish embryos. Biomedicine & Preventive Nutrition 4(2): 203-208.
- Lin S, Lai Tc, Chen L, Kwok Hf, Lau CB, Cheung PC (2014) Antioxidant and antiangiogenic properties of phenolic extract from Pleurotus tuber-regium. J Agric Food Chem 62(39): 9488-9498.
- Carvalho R, Miranda-Gonçalves V, Ferreira A, Cardoso S, Sobral A, et al. (2014) Antitumoural and antiangiogenic activity of Portuguese propolis in in vitro and in vivo Journal of Functional Foods 11: 160-171.
- Yi JM, Park JS, Lee J, Hong JT, Bang OS, et al. (2014) Anti-angiogenic potential of an ethanol extract of Annona atemoya seeds in vitro and in vivo. BMC Complement Altern Med 14: 353.
- Hernández V, Malafronte N, Mora F, Pesca MS, Aquino RP, et al. (2014) Antioxidant and antiangiogenic activity of Astronium graveolens Jacq. Leaves. Nat Prod Res 28(12): 917-922.
- Tsuzuki T, Shibata A, Kawakami Y, Nakagaya K, Miyazawa T (2007) Anti-angiogenic effects of conjugated docosahexaenoic acid in vitro and in vivo. BiosciBiotechnolBiochem 71(8): 1902-1910.
- Wihastuti T, Sargowo D, Tjokroprawiro A, Permatasari N, Widodo M, et al. (2014) Vasa vasorum anti-angiogenesis through H2O2, HIF-1α, NF-κB, and iNOS inhibition by mangosteen pericarp ethanolic extract (Garcinia mangostana Linn) in hypercholesterol-diet-given Rattus norvegicus Wistar strain. Vascular health and risk management 10: 523-531.
- Guan YY, Liu HJ, Luan X, Xu JR, Lu Q, et al. (2015) Raddeanin A, a triterpenoid saponin isolated from Anemone raddeana, suppresses the angiogenesis and growth of human colorectal tumor by inhibiting VEGFR2 signaling. Phytomedicine 22(1): 103-110.
- Kumazawa S, Kubota S, Yamamoto H, Okamura N, Sugiyamab Y, et al. (2013) Antiangiogenic activity of flavonoids from Melia azedarach. Nat Prod Commun (12): 1719-1720.
- Ke C, Wang D, Sun Y, Qiao D, Ye H, et al. (2013) Immunostimulatory and antiangiogenic activities of low molecular weight hyaluronic acid. Food Chem Toxicol 58: 401-407.
- Rudolph K, Serwe A, Erkel G (2013) Inhibition of TGF-β signaling by the fungal lactones (S)-curvularin, dehydrocurvularin, oxacyclododecindione and galiellalactone. Cytokine 61(1): 285-296.
- Xu JY, Meng QH, Chong Y, Jiao Y, Zhao L, et al. (2013) Sanguinarine is a novel VEGF inhibitor involved in the suppression of angiogenesis and cell migration. Mol Clin Oncol 1(2): 331-336.
- De Stefano I, Raspaglio G, Zannoni GF, Travaglia D, Prisco MG, et al. (2009) Antiproliferative and antiangiogenic effects of the benzophenanthridine alkaloid sanguinarine in melanoma. Biochem Pharmacol 78(11): 1374-1381.
- Eun JP, Koh GY (2004) Suppression of angiogenesis by the plant alkaloid, sanguinarine. Biochem Biophys Res Commun 317(2): 618-624.
- Lavaud A, Richomme P, Litaudon M, Andriantsitohaina R, Guilet D (2013) Antiangiogenic tocotrienol derivatives from Garcinia amplexicaulis. J Nat Prod 76(12): 2246-2252.
- Yang J, He S, Li S, Zhang R, Peng A, et al. (2013) In vitro and in vivo antiangiogenic activity of caged polyprenylated xanthones isolated from Garcinia hanburyi Hook. f. Molecules 18(12): 15305-15313.
- Li M, Wu S, Liu Z, Zhang W, Xu J, et al. (2012) Arenobufagin, a bufadienolide compound from toad venom, inhibits VEGF-mediated angiogenesis through suppression of VEGFR-2 signaling pathway. Biochem Pharmacol 83(9): 1251-1260.
- Lee B, Kim KH, Jung HJ, Kwon HJ (2012) Matairesinol inhibits angiogenesis via suppression of mitochondrial reactive oxygen species. BiochemBiophys Res Commun 421(1): 76-80.
- Nepal M, Choi HJ, Choi BY, Kim SL, Ryu JH, et al. (2012) Anti-angiogenic and anti-tumor activity of Bavachinin by targeting hypoxia-inducible factor-1α. Eur J Pharmacol 691(1-3): 28-37.
- Toyang NJ, Wabo HK, Ateh EN, Davis H, Tane P, et al. (2012) In vitro anti-prostate cancer and ex vivo antiangiogenic activity of Vernonia guineensisBenth. (Asteraceae) tuber extracts. J Ethnopharmacol 141(3): 866-671.
- Wang N, Wang ZY, Mo SL, Loo TY, Wang DM, et al. (2012) Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat 134(3): 943-955.
- Piaru SP, Mahmud R, Abdul Majid AM, Mahmoud Nassar ZD (2012) Antioxidant and antiangiogenic activities of the essential oils of Myristica fragrans and Morindacitrifolia. Asian Pac J Trop Med 5(4): 294-298.
- Bhat TA, Nambiar D, Pal A, Agarwal R, Singh RP (2012) Fisetin inhibits various attributes of angiogenesis in vitro and in vivo--implications for angioprevention. Carcinogenesis 33(2): 385-393.
- Fernand VE, Losso JN, Truax RE, Villar EE, Bwambok DK, et al. (2011) Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro. Chem Biol Interact 192(3): 220-232.
- He ZH, Zhou R, He MF, Lau CB, Yue GG, et al. (2011) Anti-angiogenic effect and mechanism of rhein from RhizomaRhei. Phytomedicine 18(6): 470-478.
- Nassar ZD, Aisha AF, Ahamed MB, Ismail Z, Abu-Salah KM, et al. (2011) Antiangiogenic properties of Koetjapic acid, a natural triterpene isolated from SandoricumkoetjaoeMerr. Cancer Cell Int 11(1): 12.
- Shengule SR, Loa-Kum-Cheung WL, Parish CR, Blairvacq M, Meijer L, et al. (2011) A one-pot synthesis and biological activity of ageladine A and analogues. J Med Chem 54(7): 2492-2503.
- Shin J, Lee HJ, Jung DB, Jung JH, Lee HJ, et al. (2011) Suppression of STAT3 and HIF-1 alpha mediates anti-angiogenic activity of betulinic acid in hypoxic PC-3 prostate cancer cells. PLoS One 6(6): e21492.
- Dehelean CA, Feflea S, Ganta S, Amiji M (2011) Anti-angiogenic effects of betulinic acid administered in nanoemulsion formulation using chorioallantoic membrane assay. J Biomed Nanotechnol 7(2): 317-324.
- Mukherjee R, Jaggi M, Rajendran P, Siddiqui MJ, Srivastava SK, et al. (2004) Betulinic acid and its derivatives as anti-angiogenic agents. Bioorg Med Chem Lett 14(9): 2181-2184.
- Chen J, Wang J, Lin L, He L, Wu Y, et al. (2012) Inhibition of STAT3 signaling pathway by nitidine chloride suppressed the angiogenesis and growth of human gastric cancer. Mol Cancer Ther 11(2): 277-287.
- López-Jiménez A, García-Caballero M, Medina MÁ, Quesada AR (2013) Anti-angiogenic properties of carnosol and carnosic acid, two major dietary compounds from rosemary. Eur J Nutr 52(1): 85-95.
- Zhang Y, Zhang H, Chen J, Zhao H, Zeng X, et al. (2012) Antitumor and antiangiogenic effects of GA-13315, a gibberellin derivative. Invest New Drugs 30(1): 8-16.
- Pang X, Yi Z, Zhang J, Lu B, Sung B, et al. (2019) Correction: Celastrol Suppresses Angiogenesis-Mediated Tumor Growth through Inhibition of AKT/Mammalian Target of Rapamycin Pathway. Cancer Res 79(3): 685.
- Zhou YX, Huang YL (2009) Antiangiogenic effect of celastrol on the growth of human glioma: an in vitro and in vivo Chin Med J (Engl) 122(14): 1666-1673.
- Huang Y, Zhou Y, Fan Y, Zhou D (2008) Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett 264(1): 101-106.
- Kanjoormana M, Kuttan G (2010) Antiangiogenic activity of ursolic acid. Integr Cancer Ther 9(2): 224-235.
- Cárdenas C, Quesada AR, Medina MA (2004) Effects of ursolic acid on different steps of the angiogenic process. BiochemBiophys Res Commun 320(2): 402-408.
- Dong Y, Lu B, Zhang X, Zhang J, Lai L, et al. (2010) Cucurbitacin E, a tetracyclic triterpenes compound from Chinese medicine, inhibits tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling pathway. Carcinogenesis 31(12): 2097-2104.
- Tan SM, Li F, Rajendran P, Kumar AP, Hui KM, et al. (2010) Identification of beta-escin as a novel inhibitor of signal transducer and activator of transcription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis inhuman hepatocellular carcinoma cells. J Pharmacol Exp Ther 334(1): 285-293.
- Harikumar KB, Sung B, Tharakan ST, Pandey MK, Joy B, et al. (2010) Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol Cancer Res 8(5): 751-761.
- Jung HJ, Shim JS, Lee J, Song YM, Park KC, et al. (2010) Terpestacin inhibits tumor angiogenesis by targeting UQCRB of mitochondrial complex III and suppressing hypoxia-induced reactive oxygen species production and cellular oxygen sensing. J Biol Chem 285(15): 11584-11595.
- Lopes FC, Rocha A, Pirraco A, Regasini LO, Silva DH, et al. (2009) Anti-angiogenic effects of pterogynidine alkaloid isolated from Alchorneaglandulosa. BMC Complement Altern Med 9: 15.
- Hussain S, Slevin M, Matou S, Ahmed N, Choudhary MI, et al. (2008) Anti-angiogenic activity of sesterterpenes; natural product inhibitors of FGF-2-induced angiogenesis. Angiogenesis 11(3): 245-256.
- Oh SH, Woo JK, Jin Q, Kang HJ, Jeong JW, et al. (2008) Identification of novel antiangiogenic anticancer activities of deguelin targeting hypoxia-inducible factor-1 alpha. Int J Cancer 122(1): 5-14.
- Yoysungnoen P, Wirachwong P, Changtam C, Suksamrarn A, Patumraj S (2008) Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J Gastroenterol 14(13): 2003-2009.
- Li L, Ahmed B, Mehta K, Kurzrock R (2007) Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol Cancer Ther 6(4): 1276-1282.
- Bae MK, Kim SH, Jeong JW, Lee YM, Kim HS, et al. (2006) Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep 15(6): 1557-1562.
- Martínez-Poveda B, Quesada AR, Medina MA (2005) Hyperforin, a bio-active compound of St. John's Wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int J Cancer 117(5): 775-780.
- Singh Rana P, Rajesh Agarwal (2003) Tumor angiogenesis: a potential target in cancer control by phytochemicals. Current cancer drug targets 3(3): 205-217.
- Samman Munir, Asad A Shah, Muhammad Shahid, Muhammad S Ahmed, Aqsa Shahid, et al. (2020) Anti-angiogenesis Potential of Phytochemicals for the Therapeutic Management of Tumors. Current Pharmaceutical Design 26(2): 265-278.
- Altaf Ahmad Shah, Mohammad A Kamal, Salman Akhtar (2021) Tumor Angiogenesis and VEGFR-2: Mechanism, Pathways and Current Biological Therapeutic Intervention. Current Drug Metabolism 21(1): 50-59.
- Bialas, P Kafarski (2009) Proteases as Anti-Cancer Targets - Molecular and Biological Basis for Development of Inhibitor-Like Drugs Against Cancer. Anti-Cancer Agents in Medicinal Chemistry 9(7): 728-762.
- Heike E Daldrup-Link, Gerhard H Simon, Robert C Brasch (2006) Imaging of Tumor Angiogenesis: Current Approaches and Future Prospects. Current Pharmaceutical Design 12(21): 2661-2672.
- Virginia Di Paolo, Marta Colletti, Valentina Ferruzzi, Ida Russo, Angela Galardi, et al. (2020) Circulating Biomarkers for Tumor Angiogenesis: Where Are We?. Current Medicinal Chemistry 27(14): 2361-2380.
- Mark N Kirstein, Megan M Moore, Arkadiusz Z Dudek (2006) Review of Selected Patents for Cancer Therapy Targeting Tumor Angiogenesis. Recent Patents on Anti-Cancer Drug Discovery 1(2): 153-161.

 Review Article
Review Article