Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Rong Cao, Haofei Hu and Qijun Wan*
Received: July 07, 2022; Published: July 15, 2022
*Corresponding author: Qijun Wan, Department of Nephrology, The First Affiliated Hospital of Shenzhen University the Second People’s Hospital of Shenzhen, Shenzhen, 518035, China
DOI: 10.26717/BJSTR.2022.45.007165
Aims: We examined the relationship between the hemoglobin (Hb) level and estimated glomerular filtration rate (eGFR) in patients with type 2 diabetes mellitus (T2DM).
Methods: This cross-sectional study recruited a total of 139 patients with T2DM and diabetic kidney diseases. Various demographic and clinical characteristics at baseline (age, sex, BMI, duration of diabetes, systolic/diastolic blood pressure, Fasting blood glucose, HbA1c, serum albumin, Uric acid, Triglyceride, Total cholesterol, LDL-C, 24-h protein excretion, serum creatinine, eGFR and antidiabetic therapy) were examined. The relationship between hemoglobin (Hb) level and eGFR was examined by Pearson’s correlation analyses. Multivariate regression analyses were used to assess the association of hemoglobin (Hb) level with eGFR.
Results: A positive correlation was found between hemoglobin and eGFR (r =0.1564, p<0.0001); A negative correlation was found between hemoglobin and Serum creatinine (r =-0.1613, p<0.0001). The baseline hemoglobin (Hb) level and baseline eGFR have liner relationship. Multivariate regression analyses showed that eGFR was associated with hemoglobin, estimates β(CI) was 0.45 (0.21, 0.68), 0.41 (0.18, 0.63), 0.48 (0.16, 0.79) in Non-adjusted, Multivariate-Adjust Model I and Multivariate-Adjust Model II, respectively.
Conclusions: The hemoglobin level at baseline is an independent protective factor of baseline eGFR, and monitoring of hemoglobin can play an important role in management of diabetic nephropathy.
Keywords: Baseline Hemoglobin; Baseline eGFR; Diabetic Kidney Disease; Type 2 Diabetes Mellitus
The incidence and prevalence of diabetes mellitus (DM) continue to grow markedly throughout the world, due primarily to the increase in type 2 DM (T2DM) [1,2]. And the prevalence of diabetic nephropathy has increased dramatically, surpassing glomerulonephritis, becoming the first cause of ESRD (End -Stage Renal Disease) [3]. ESRD has caused great harm and burden to families and society because of its high fatality rate and high cost. So, it is important to find the factors that influence the occurrence and development of ESRD. As we all known the lower baseline eGFR (estimated Glomerular Filtration Rate), the faster development of kidney disease [4], which means that it is crucial to find the index that associated with the decline of eGFR. Anemia is a common complication of chronic kidney disease [5,6]. And it is reported that anemia is associated with an increased risk for progression to ESRD in diabetic and nondiabetic adults with chronic kidney disease (CKD) and anemia is associated with increased morbidity and mortality in patients with end-stage renal disease [7-11]. It is reported that the severity of anemia depends on renal function, the worse the renal function, the more severe of anemia [12]. Diabetesrelated anemia has been found in diabetic nephropathy uremia patients. However, recent studies showed serum erythropoietin was lower even in type 1 or type 2 diabetes patients without overt uremia [13,14]. Patients with diabetic nephropathy may have worse clinical outcomes related to anemia than other etiologies of kidney failure. Hemoglobin level are independent risk factors that predict renal outcomes [15], but the relationship between hemoglobin and baseline eGFR is unknown. So, we would like to examine the relationship between the hemoglobin (Hb) level and estimated glomerular filtration rate in patients with type 2 diabetes mellitus.
This is an observational cross-sectional study. All study patients had signed the consent form when hospitalized, and the research was approved by the ethics committee of The First Affiliated Hospital of Shenzhen University. The baseline information of the subjects was all collected from the First Affiliated Hospital of Shenzhen University database.
This study recruited a total of 139 patients with T2DM and diabetic kidney diseases in The First Affiliated Hospital of Shenzhen from 2012 to 2017, The diagnosis of T2DM and DN (Diabetic Nephropathy) was based upon the criteria proposed by the American Diabetes Association (ADA) in 2017 [16] and the Renal Pathology Society in 2010 [17]. Exclusion criteria were concomitant with acute kidney disease (7) or combined with other kidney diseases such as membranous nephropathy (2) or IgA nephropathy (4), no information of hemoglobin (3) or having entered ESRD before hospitalization. And finally, a total of 123 patients were enrolled in this study. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Shenzhen University. Written informed consent was obtained from all participants.
The Baseline clinical information of patients was gathered at the time of the first hospitalization during the study period, including age, sex, BMI (Body Mass Index), duration of diabetes, systolic/ diastolic blood pressure(SBP/DBP), Fasting blood glucose(FBG), HbA1c ( Hemoglobin A1c), serum albumin, Uric acid, Triglyceride, Total cholesterol, LDL-C (Low-Density Lipoprotein-Cholesterol), 24-h protein excretion, serum creatinine, estimated glomerular filtration rate (eGFR) (ml/min/1.73 m2), and Antidiabetic therapy. The eGFR was computed according to the Modification of Diet in Renal Disease equation.
All analyses were performed with Empower Stats software (www.empowerstats.com) and R software. Continuous variables were presented as the mean with standard deviation (SD) or the median with range, and differences in means were compared using Student’s t test or the Mann–Whitney test, as appropriate. Categorical variables were expressed as counts and ratios, and the differences in proportions were analyzed using the Chi-square test. The influence of those variables on eGFR was evaluated by a multiple linear regression model. The relationship between hemoglobin and eGFR was assessed using Cox regression. Multivariate Cox analysis was applied to pinpoint the independent risk factors of prognosis. A two-sided p value < 0.05 was reckoned to be statistically significant.
A total of 123 T2DM patients were equally distributed to two group according to median hemoglobin level, there were 59 in Hb <107 g/L group and 64 in Hb≥107 g/L, respectively. The demographic and clinical characteristics of the analyzed participants including age, sex, BMI, duration of diabetes, systolic/ diastolic blood pressure, Fasting blood glucose, HbA1c, serum albumin, Uric acid, Triglyceride, Total cholesterol, LDL-C, 24-h protein excretion, serum creatinine, eGFR and Antidiabetic therapy in Table 1. Of note, eGFR was much higher in the Hb≥107 g/L group than in the Hb <107 g/L group (P < 0.001). In addition, the patients in the Hb≥107 g/L group were more male sex, older and had higher level of BMI, serum albumin, Fasting blood glucose, HbA1c, Triglyceride. However, compared with the Hb <107 g/L group, patients in Hb≥107 g/L group had lower level of serum creatinine and 24-h protein excretion. There were more people choose Oral antidiabetic drugs rather than Insulin to lowing blood glucose in Hb≥107 g/L group.
A positive correlation was found between hemoglobin and eGFR (r =0.1564, p<0.0001) (Figure 1a); A negative correlation was found between hemoglobin and Serum creatinine (r =-0.1613, p<0.0001) (Figure 1b) (Table1).
Figure 1: The Correlation between hemoglobin and renal function in diabetic kidney disease patient.
A. Correlation between hemoglobin and eGFR in patients with type 2 diabetes.
B. Correlation between hemoglobin and Serum creatinine in patients with type 2 diabetes.
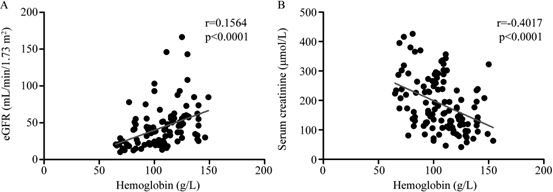
Table 1: The demographic and clinical characteristics of the 123 Chinese patients with diabetic kidney disease at baseline.
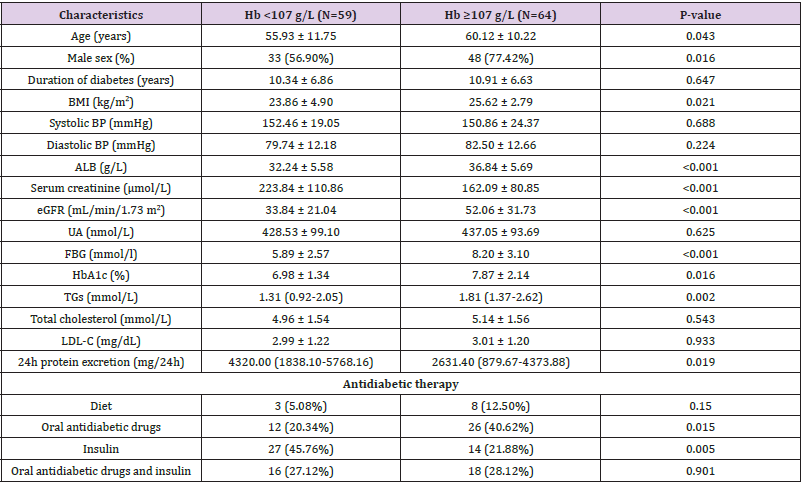
Note: Values are expressed as the mean ± SD, number (percent) or median (interquartile range). eGFR, estimated glomerular filtration rate (MDRD). This table is generated using empower stats (www.empowerstats.com) and R software.
Table 2 presents the association between eGFR and other baseline characteristics with no adjustment. Consistent with the existing research literature, higher FBG, HbA1c and serum albumin were each associated with better eGFR, whereas higher Systolic BP, serum creatinine and more 24-h protein excretion were associated with worse eGFR. Age, sex, BMI, TGs and total cholesterol were not significantly associated with eGFR in this study population.
Table 3 displays the results of multivariate regression for effects of hemoglobin on eGFR. eGFR was associated with hemoglobin in crude model, and the unadjusted estimates β (95% confidence interval [CI]) was 0.45 (0.21, 0.68), and this relationship remained statistically significant after adjusting for Age (years), Systolic BP (mmHg) and duration of diabetes (years), with β (95% CI) of 0.41 (0.18, 0.63) in Multivariate-Adjust Model I. What’s more, when adjusted for Age, Systolic BP, duration of diabetes, ALB, FBG, HbA1c and proteinuria in Multivariate-Adjust Model II, the β (95% CI) was 0.48 (0.16, 0.79). Hemoglobin was a protective factor of baseline eGFR in all these 3 models. After hemoglobin was divided into low, middle and high 3 group according to hemoglobin level, in middle hemoglobin group, the β (95% CI) was 8.32 (-4.07, 20.71), 5.97 (-6.18, 18.13) and 6.41 (-17.74, 30.55) respectively; in high hemoglobin group, the β (95% CI) was 27.46 (15.15, 39.77), 24.96 (12.77, 37.15) and 19.41 (-8.06, 46.88) respectively. It indicates that the relationship between hemoglobin and baseline eGFR maybe linear relationship. Subgroup analyses are present in Table 4. We found no significant heterogeneity among analyzed subgroups according to sex (male or female), age (<50 or >=50-year old), Systolic BP ((<140 or >=140 mmHg) , FBG (<7 or >=7mmol/l), HbA1c (<6.5 or >=6.5%), ALB (<35 or >=35g/L), 24h protein excretion (<3500 or >=3500mg/24h), duration of diabetes (low or middle or high),TGs (low or middle or high) , Total cholesterol (low or middle or high), LDL-C (low or middle or high).
Table 3: Multivariate regression for effect of hemoglobin on eGFR in diabetic kidney disease patient.
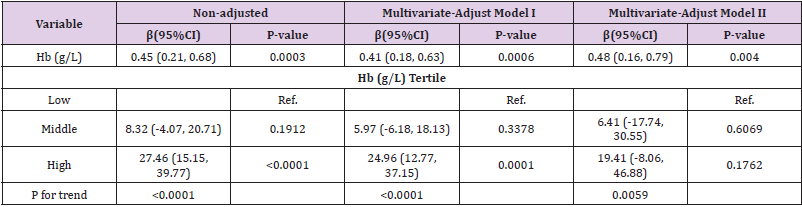
Note: Non-adjusted model adjustfor: None; Multivariate-Adjust Model I adjust for: Age (years); Systolic BP (mmHg); duration of diabetes (years); Multivariate-Adjust Model II adjust for: Age (years); Systolic BP (mmHg); duration of diabetes (years); ALB (g/L); FBG (mmol/l); HbA1c (%); proteinuria (mg/24h).
In the present study, we examine the relationship between the baseline hemoglobin (Hb) level and estimated glomerular filtration rate (eGFR) in patients with type 2 diabetes mellitus. We speculate the higher of the baseline hemoglobin (Hb) level and the better of estimated glomerular filtration rate (eGFR) .And our data show that baseline hemoglobin and eGFR had a positive correlation(r =0.1564, p<0.0001), while baseline hemoglobin and Serum creatinine had a negative correlation (r =-0.1613, p<0.0001); what’s more, the baseline hemoglobin (Hb) level and baseline eGFR have liner relationship, and eGFR was associated with hemoglobin, estimates β(CI) was 0.48 (0.16, 0.79) by multivariate regression analyses. Mohanram, A. shown that patients with hemoglobin concentration at baseline less than 120g/l had lower glomerular filtration rate, both in Losartan and Placebo group [18], which agrees with our results, we also found that the lower of the baseline Hb level, the worse of the baseline eGFR, and what’s more, they are linear relationship. However, Mohanram, A’s data was just a descriptive study data, and the author didn’t adjust the related mixed factors, what’s more, no report has investigated the relationship between the baseline hemoglobin (Hb) level and baseline eGFR by multivariate regression analyses. However, we use the Non- Adjust and 2 Multivariate-Adjust Models to analysis the relationship between baseline hemoglobin concentration and baseline eGFR, and we find the liner relationship between the baseline hemoglobin (Hb) level and baseline eGFR, the lower of the baseline Hb level, the worse of the baseline eGFR, In our study, the hemoglobin level at baseline is an independent protective factor of baseline eGFR, multivariate regression analyses indicate eGFR is associated with hemoglobin, estimates β(CI) is 0.48 (0.16, 0.79).
There were many studies investigated the relationship between baseline hemoglobin and the follow-up eGFR. Mohanram, A found that even mild anemia, Hb <13.8 g/dL increases risk for progression to ESRD and hemoglobin is an independent risk factor for progression of nephropathy to ESRD in type 2 diabetes [11]. Kuriyama, S found anemia was a factor in the progression of end-stage renal failure, and reversal of anemia by EPO can retard the progression of renal failure [19]. Keane, W.F also found that after control of blood pressure in type 2 diabetic patients with nephropathy, hemoglobin level was still an independent risk factor that predict renal outcomes [5]. Rossert, J. found that correction of anemia with epoetin may slow the rate of progression of renal failure [20]. These results indicate that baseline hemoglobin concentration was an independent risk factor for progression of ESRD and death. What’s more, Wei Yang found greater hemoglobin variability is independently associated with higher mortality, the data shown after adjusting for multiple covariates, each 1g/dl increase in the residual standard deviation was associated with a 33% increase in rate of death [21]. However, there was no study focus on the baseline hemoglobin concentration and the baseline eGFR. In our study, we are interested in the relationship between baseline hemoglobin concentration and the baseline eGFR, and we find baseline eGFR was associated with baseline hemoglobin, the estimates β(CI) was 0.48 (0.16, 0.79) by multivariate regression analyses. But we did not investigate the relationship between baseline hemoglobin and the follow-up eGFR in our study.
Our study is novel from two perspectives. One perspective is that we found the liner relationship between the baseline hemoglobin (Hb) level and baseline eGFR by multivariate regression analyses. Another perspective is that we investigated the baseline hemoglobin concentration and the baseline eGFR but not the follow-up eGFR. This study has some limitations that should be mentioned. First, our study is a cross-sectional design; Second, because of the limited number of patients in this study, the existence of biases and confounding factors cannot be ruled out. Third, we did not investigate the relationship between baseline hemoglobin and the follow-up eGFR in our study. Therefore, further prospective studies with larger sample sizes are required to confirm the present findings, and the relationship between baseline hemoglobin and the follow-up eGFR need to be confirmed.
In conclusion, the baseline hemoglobin (Hb) level and baseline eGFR have liner relationship, and the hemoglobin level at baseline is an independent protective factor of baseline eGFR, and monitoring of hemoglobin can play an important role in management of diabetic nephropathy.
This work was supported by the Natural Science Foundation of Guangdong Province (2021A1515010991) and the Shenzhen Science and Technology Project (JCYJ20210324103602006). This work was also supported by the Shenzhen Key Medical Discipline Construction Fund (SZXK009).


