Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gisela González Hein1*, Carlos González2, Isabel Aguirre3 and Bernardo Huaracán1
Received: July 20, 2022; Published: July 27, 2022
*Corresponding author: Gisela González Hein, Bioingentech, Santiago, 8330243, Chile
DOI: 10.26717/BJSTR.2022.45.007189
Two epornitics of avian pox occurred in psittacine birds, seriously affecting redrumped parrots (Psephotus haematonotus). Avipoxvirus (APV) infection could be characterized in the Psephotus necropsied by diphtheric, cutaneous and systemic forms. P. haematonotus were affected by splenitis and focal necrotic hepatitis attributed to Avipoxvirus. The DNA sequences of the Pb4 gene were determined for 4 Avipoxvirus positive birds. We present a phylogeny of all available partial sequences of psittacine birds including two novel Avipoxvirus of this study (GeneBank KY748237 and KY748236). The nucleotide sequences clearly grouped within the clade C of the Psittacinepox virus (PSPV). We conclude that these infections in Psephotus were caused by an avian poxvirus previously unrecognized in Chile. Further characterization of PSPV and the development of an urgent generation of effective and safe vaccines are needed in psittacine birds.
Keywords: Avipoxvirus; Poxviridae; Psittacine Birds; Psittacinepox Virus; Sistemic Form; Avian Pox
Viruses of the genus Avipoxvirus (Family: Poxviridae) affect a great diversity of avian species (more than 250 species). Avipoxvirus (APV) causing proliferative lesions in captive and wild bird species worldwide. Currently, ten species make up the genus, including Psittacinepox virus (PSPV). These Avipoxvirus (APV) of psittacine birds appear to represent a third clade similarly divergent as that of canarypox and fowlpox [1]. Histopathological lesions include necrosis of the liver and heart, as well as air sacculitis, pneumonia, peritonitis, and accumulation of necrotic debris on the surface of the alimentary tract. Characteristic intracytoplasmic inclusión bodies (Bollinger bodies) may be noted in lesions in the mucosa of the sinuses, trachea, crop, esophagus or throat [2]. Viral cultivation in chorioallantoic membrane (CAM) of embryonated chicken eggs and in cell lines together with electron microscopy are available methods to isolate or detect the virus replication [3]. In bird species, virus neutralization, ELISA, immunodiffusion on agar gel and hemagglutination- inhibition test are serologic tests that can also be used to diagnose poxvirus infection in the host [3,4]. To date, the most sensitive and fast techniques are associated with molecular approaches (Manarolla, et al. [5]). Conventional and real time polymerase chain reaction (PCR) techniques provide results more rapidly than virus isolation. Thus, recently, PSPV infection could be confirmed by commercially available PCR kits.
Hence, the amplification of a region of P4b core protein gene sequence, a highly conserved gene of APV, is commonly used to diagnose APV infections, amongst all poxviruses (Binns, et al. [6]). The P4b gene has also been reported in phylogenetic studies to distinguish among the different clades A, B, C, A1-7 and B1-3 of APV (Jarmin, et al. [7-10]). Three main clades (A to C) are differentiated within the genus APV; being clade A known as the fowlpox clade, clade B as the canarypox clade, and clade C as the psittacinepox virus clade (Gyuranecz, et al. [9]). Two additional clades (D and E) have also been proposed in poultry (Manarolla, et al. [5,11,12]). To date, there is little information about APV infections in Psittaciformes birds (Bolte, et al. [13]). In 2016, a disease that mainly affected “Psephotus haematonotus” in an aviary (A) of the Metropolitan Region (Chile) was investigated in one P. haematonotus (2 months old) and whose lesions were associated with APV infection. On the other hand, between 2016 and 2017, cases of disease with signology compatible with APV were investigated in another aviary (B) of Chile, in two dead P. haematonotus sent to our laboratory. The objective of this study was to histopathologically characterize the lesions generated by these avian pox isolates of psittacine birds of Chile. Additionally, molecular determination with PCR and a phylogenetic analysis of the sequences obtained were carried out.
Diverse birds of the order Psittaciformes, from two different aviaries, presented lethargy, blepharitis, thickening of the eyelids and dyspnea. Samples taken from the eye, trachea, liver and spleen of four dead P. haematonotus were fixed in 10% buffered formalin. Multiple samples were embedded in paraffin, sectioned, and stained with hematoxylin and eosin, according to standard protocols. A blood sample from one live Barnadius zonarius between 5 and 6 weeks of age also affected was sent for analysis. An APV infection was suspected on the basis of clinical presentations, necropsy and histopathological findings in 4 dead psittacine birds. Also, we processed tissues for molecular analysis to confirm the clinical and pathology-based diagnosis. Samples of fresh tissue from affected eyelids and tracheas were obtained to perform DNA extraction, according to the manufacturer’s instructions of the commercial kit for APV (Bioingentech, Concepcion, Chile). A PCR was performed with specific primers of the avian pox kit of Bioingentech, which amplifies a 452 bp partial sequence of the gene that codes for the central protein P4b of the APV. The PCR program consisted of initial denaturation at 94 ºC for 3 minutes, then 30 cycles with 30 seconds for denaturation at 94 ºC, 30 seconds annealing at 57 ºC and 30 seconds extension at 72 ºC followed by a final extension step 5 minutes at 72 ºC. PCR products were subjected to electrophoresis on a 1.5% agarose gel containing a fluorescent nucleic acid marker. In addition, we used the PCR system based on the known Fowlpox virus DNA polymerase gene sequence (Binns et al., 1989) utilizing the primer pair: PoPr1, 5′-CGCCGCATCATCTACTTATC-3′; and PoPr2, 5′-CCACACAGCGCCATTCATTA-3′ knowing this method is not able to detect all poxvirus strains (Gyuranecz, et al. [9]).
Detection of other pathogens: each dead bird was checked for the presence of DNA from common pathogens of psittacine birds such as Aves Polyomavirus 1, Pacheco’s disease virus, and Beak and Feather disease virus using Bioingentech PCR kits. Feathers and cloacal swabs were collected. In order to rule out differential diagnoses, we subjected nucleic acids extracted from freshly collected specimens (eyelids and tracheas) to a PCR assay for detection of Mycoplasma gallisepticum, Avibacterium paragallinarum, Trichomonas gallinae, Pasteurella multocida, Staphylococcus aureus y Chlamydophila psittaci) using Bioingentech PCR kits. Sequence analysis: the PCR products were automatically sequenced in both directions at Pontifical Catholic University of Chile. Sequencing was done on an ABI PRISM® 3130 Applied Biosystems machine. The APV sequences obtained (accession numbers: KY748237 and KY748236) were subjected to multiple comparisons together with international sequences available in GenBank that included representatives from different avian species, countries, and clades and subclades reported for Avipoxvirus, namely, fowlpox clade (A), canarypox clade (B), and psittacinepox clade (C). In total, 10 sequences of APV described from psittacine birds (Gyuranecz, et al. [9,14-17]) were included and analyzed together with the two sequences of the present study (Table 1). For this purpose, a multiple sequence alignment tool of Clustal Omega was used (Larkin, et al. [18-20]).
Table 1: List of nucleotide sequences of Avipoxvirus of Gen Bank described in Psittaciformes.
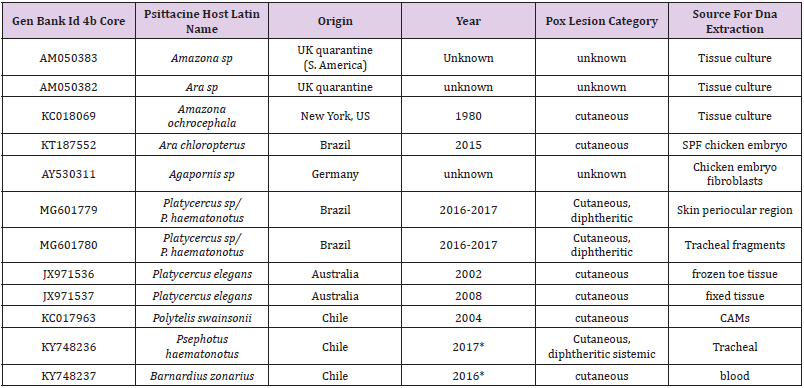
Note: *This study
Once aligned, an area of 428 nucleotide positions of the 4b core gene from a total of 29 sequences of APV strains were subjected to phylogenetic analysis using the Maximum likelihood (ML) method and Jukes and Cantor model [21]. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 2.0258)). The evolutionary analysis was conducted in MEGA7 software (Kumar et al., 2016).
The virus caused a highly infectious disease that produced up to 100% morbidity and mortality in affected P. haematonotus. Aviary A (2016) and B (2016-2017). Four P. haematonotus revealed histopathological lesions: blepharitis and/or ulcerative necrotic lesions in conjunctival mucosa associated with viral cytopathic effect of the type described for avian pox. Three P. haematonotus presented tracheitis with an abundant deposit of amorphous necrotic material with intense desquamation of epithelial cells. In the remnant epithelia of the trachea, Borrel and Bollinger bodies were observed. In two of them we also reported the presence of splenitis and focal necrotic hepatitis with the presence of intranuclear inclusions of the viral type associated with APV.
Specifically, two Psephotus liver tissue samples showed intense tumefaction of the hepatocytes that were swollen and with vacuolated cytoplasm. In some areas disseminated and coalescent foci of hepatocyte necrosis were observed. Disseminated microhemorrhage foci were observed in peripheral areas. Occasionally, periportal mononuclear infiltration with intense congestion and intense swelling of hepatocytes with disseminated foci of necrosis and microhemorrhage of hepatic parenchyma were observed. In some cases, along with intense periportal lymphocytic infiltration, accumulation of biliary pigment was seen in hepatocytes. Associated with areas of hepatic necrosis, the presence of numerous eosinophilic inclusions of the viral type (Borrel Bodies) was detected. In two of the remaining birds, liver congestion and swelling were also observed with isolated necrosis foci, but no viral eosinophilic inclusions of the Borrel body type were observed in the liver tissue.
The splenic tissue samples in two Psephotus showed hemorrhage foci with fibrin deposits and erythrocyte lysis, as well as numerous macrophages loaded with hemosiderin. There is also a displacement of white pulp lymphoid tissue by hemorrhagic zones and an accumulation of macrophages with abundant phagocytosed hemosiderin granules. Associated with areas of necrosis, the presence of numerous eosinophilic inclusions of the Borrel type was detected, forming large intracytoplasmic conglomerate Bollinger bodies. The distribution of lesions described in these 2 out of 4 cases confirms the wide variation that occurs in poxvirus infections in avian species. Specific genomic fragments of Avipoxviruses from all samples of birds examined were amplified using PCR reaction. We did not amplify fragments in the samples using the pair of primers PoPr1 and PoPr2 described for Fowlpox virus strains. Pathogens considered in the differential diagnoses were also excluded using PCR in dead birds. All samples collected were negative for other pathogens (Mycoplasma gallisepticum, Avibacterium paragallinarum, Pasteurella multocida, Trichomonas gallinae, Staphylococcus aureus y Chlamydophila psittaci) by the molecular assays used. A blood sample of a Barnardius zonarius was also positive to Beak and feather disease virus. Three partial DNA sequences from the gene encoding 4b core protein of avipoxviruses were obtained from eyelid and tracheal lesions of a dead P. haematonotus and one sequence from a blood sample of a Barnardius zonarius from the aviary (A). This last bird was alive at the time of sampling, with clinical signs of poxvirus infection and a detectable viremia. This bird was located in a cage next to the P. haematonotus and died later. These 4 nucleotide sequences were obtained and sequenced.
The three partial sequences of the P4b gene obtained from the P. haematonotus generated an identical genetic profile. Two of the 4 Avipoxvirus partial nucleotide sequences generated in this study were deposited in GenBank (Accession numbers: KY748237 for B. zonarius and KY748236 for P. haematonotus). Similarity and phylogenetic analysis revealed that both partial sequences of this study were identical with nucleotide sequence strains AM050383 CVL 364/89 (Amazona parrot), AM050382 (macaw) both from psittacine birds in quarantines in The United Kingdom, AY530311 APIII (Agapornis sp. in Germany), KC018069 P110 (Amazona spp, New York), and MG601780, MG601779 and KT187552 these last coming from three Brazilian parrots). All these sequences clearly grouped within the clade of the PSPV described so far. In multiple comparison analyses, the highest percentage of similarity was found with sequences from psittacine birds belonging to clade C (99,6-100%). The similarity with sequences from clade A ranged between 75-77% and from clade B, between 74-75.6 (data not shown). The nucleotide sequence of an APV isolated from a superb parrot originating from Chile clustered in subclade A1, and partial sequences of APV isolated from two Platycercus elegans (JX971537, JX971536) originating from Australia were grouped in a different clade from A, B or C. Interestingly, these two partial sequences showed very low percentages of similarity with clade C (39.9- 41.2%, data not shown) but also with both clade A and B.
The APV infections showed a high virulence in P. haematonotus and in both aviaries the birds that had contact with the Psephotus were affected and died (12 Polytelis spp., 30 Platycercus spp. and young birds: 2 Phyrrhura molinae, 2 Ara spp and 4 Barnardius zonarius). However, almost every South American parrots and Psittaculas spp. and Agapornis spp. were asymptomatic in both aviaries. Similar to what happened in South America the outbreak in exotic psittacines in southern Brazil, the Australian species and P. haematonotus, were the most affected (Murer, et al. [17]). Four clinicopathological presentations are commonly described in birds: a skin (dry) form with cutaneous wart-like lesions on unfeathered skin, a diphtheritic (wet) form in which lesions can be found in the upper gastrointestinal and respiratory tracts, a systemic form and an oncogenic form (Gerlach, et al. [22-25]). A severe viraemic (septicaemic) form of the disease in canaries also has been described (Shivaprasad, et al. [26]). In other cases, the infection may be localized and characterized by nodules in some internal organs (Mete, et al. [27]). Outbreak of a combined cutaneous and diphtheritic forms of psittacine pox also has been reported in Agapornis roseicollis (Dan-Yuan, et al. [28]).
Figure 1: Maximum-likelihood phylogeny generated from partial sequences from 4b core gene of Avipoxvirus. The phylogenetic relationships revealed grouping with psittacine poxviruses (clade C), including isolates from AM050382, AM050383, KC018069, AY530311, MG601779, MG601780, and KT187552). To enable comparisons, other strains were included. Avipoxvirus clades A to C, subclades, and clusters are labeled according to the nomenclature of Jarmin et al. [7] and Jarvi et al. [38]. Isolate origins are given either as U.S. state abbreviations or using the following location codes: Argentina (AR), Australia (AU), Brazil (BR), Chile (CL), Germany (DE), Hungary (HU), India (IN), Italy (IT), South Africa (ZA), Tanzania (TZ), and United Kingdom (UK). Only boostrap values over 70% are shown next to each branch. Boostraping was carried out on 1000 replicates. Evolutionary analysis were conducted in MEGA7 software.
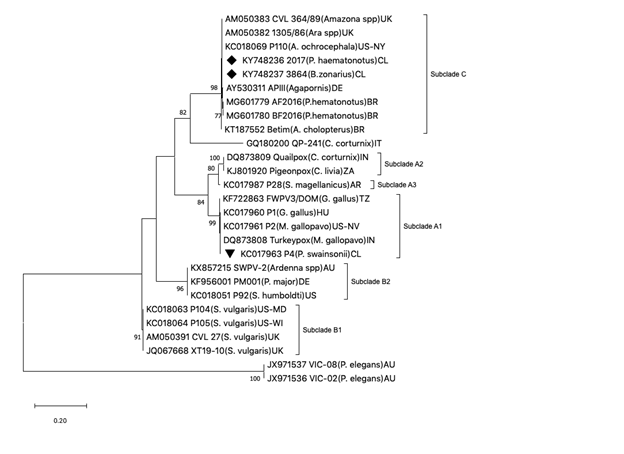
In the cases of this study a combination of skin and diphtheritic forms were observed in P. haematonotus. The systemic form also was observed in two of the necropsied Psephotus. This last form is unusual in psittacine birds and to date there is only one report of this type in Agapornis sp. (Tsai, et al. [24]). Reports of liver and spleen lesions attributed to APV have not been found in Psittaciformes birds. However, spleen and liver damage has been reported in canaries (Serinus canaria) (Shivaprasad, et al. [26]) and in an Andean Condor (Vultur gryphus) (Kim, et al. [29]). Mortality rates are highest when diphtheritic lesions cause defects in the mucosal barrier of the alimentary and respiratory tracts, allowing secondary bacterial, fungal or chlamydial organisms (Graham, et al. [30-32]) or when cutaneous lesions around the eyes interfere with normal functions [3]. Some case reports of pox infections in psittacine species have described severe disease leading to mortality (Gerlach, et al. [22,17,33]) similar to what is reported in this study. On physical examination and pathologic findings in U. S. the effects of pox in some P. haematonutus included bilateral conjunctivitis, blepharitis, and dyspnea and a combination of skin and diphtheritic forms (Beaufrere, et al. [34,17]) also described APV of clade C in psittacine birds with cutaneous and diphtheritic form. By contrast, other reports in psittacine birds described a skin disease (cutaneous form) affecting principally the feet and head, in crimson rosellas (Platycercus elegans) in Australia and Psittacus erithacus from Saudi Arabia (Tarello, et al. [35]). The partial sequences of poxvirus JX971537, JX971536 detected from these Crimson rosellas represented a new genetic clade of avipoxviruses (Figure 1) and t.hese strains of APV were categorized with low virulence and high species specificity (Slocombe, et al. [14]).
The two partial sequences of the P4b gene of this study were submitted to Gen Bank and grouped within the clade of the PSPV, as we expected. The former poxvirus isolated from an outbreak in captive psittacine birds in Chile (Gonzalez- Hein, et al. [36]) in a Chilean Superb parrot (Gen Bank Accession numbers AB177959) was clustered in clade A, together with fowl domestic birds. In the last case there was previous information about a vaccine against fowlpox in Galliformes birds that belonged to the same aviary. There is a complex relationship between avian poxviruses and their hosts. The degree of susceptibility and the severity of the disease seem to vary among species and strains (Gyuranecz, et al. [9]). The source of APV in the aviaries probably came from a group of birds that entered the aviary without any quarantine, from the movement of birds in exhibitions without post quarantine or the introduction of imported psittacine birds. Both aviaries had been in operation for many years and did not have any prior history of APV in the psittacine birds. Cleaning and disinfection of the premises, removal of the dead birds and isolation of sick birds were successful as part of the disease control. In veterinary medicine, vaccination plays a major role in preventing viral infections (Katoh, et al. [37]). The currently available vaccines against fowlpox, canarypox, pigeon pox, and quail pox are each produced using virus strains isolated from the respective avian group [3]. There is an increasing demand for new vaccines against avian pox virus infections to help protect a wide range of birds, especially endangered species (Manarolla, et al. [5,38]). Therefore, effective and safe vaccination for these virus infections are urgently needed for psittacine birds and other orders of birds.
Recommended by the OIE. PSPV proposed measures by department of Agriculture, Water, and the Environment (Australian Government) include A pre-export and post-entry quarantine period of at least 30 days and 14 days respectively, in order to identify clinically affected birds. A requirement that the bird undergo veterinary examination during a period of pre-export quarantine and post-entry quarantine and either no lesions suggestive of avian pox are present or, if such lesions are present, they are comprehensively investigated, and avian pox is ruled out as a cause [39].
There is limited genetic information and antigenic information available on avian poxviruses in psittacine birds. The access of a significant number of complete nucleotide sequences of the genomes of psittacinepox viruses would help to understand the behavior of the virus identifying the genomic changes responsible for the pathogenicity of these PSPV variants. This information is also necessary for the generation of new vaccines, to overcome the problems related to the conservation of bird species, decreasing pox-related economic losses and the emotional cost of aviculturists associated to the loss of birds.


