Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
S Guggenbichler1* and JP Guggenbichler2
Received: April 20, 2022; Published: July 29, 2022
*Corresponding author: Guggenbichler JP, Em. Prof. Dr. med., Department or Pediatrics, Univ. Erlangen, Germany and CEO AmiSTec GMbH and Co KG, Kössen, Austria
DOI: 10.26717/BJSTR.2022.45.007200
Abbrevations: SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus; COVID-19: Coronavirus Disease; WHO: World Health Organization; PVC: Polyvinyl Chloride; ESBL: Extended-Spectrum β-Lactamase; R0: Reproduction Number; MDR: Multidrug-Resistant; HCAI: Healthcare-Associated Infections; CDC: Centers for Disease Control; QAC: Quaternary Ammonium Compounds
Public transportation is the primary, and frequently the only mode of travel for many people. By those means also pathogens are travelling with people across a wide range of countries. The transmission of viral and bacterial pathogens by public transportation is a challenging problem and requires multifaceted solutions [1,2]. Public transport vehicles e.g. airplanes as well as trains, trolleys, taxi, rental cars are confined spaces that are conductive for human-to-human transmission of infectious diseases but also from contaminated surfaces [3]. The problem however starts already at the transport hub, airports, train stations etc. and must be an integral part of the solution (Figures 1 & 2). A multitude of different microorganisms (MRSA, extended spectrum ß lactamase forming gram negative microorganisms, Carbapenem resistant Klebsiella pneumoniae, Acinetobacter) have been isolated from both surfaces. The majority of these germs were insensitive against virtually all antibiotics. Respiratory viruses spread across wide geographical areas in short periods of time, resulting in high levels of morbidity and mortality. Consequently, several countries have reported clusters of cases in public transport vehicles with infections caused by respiratory viruses including Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and COVID 19 but also by multi-resistant microorganisms [4-6]. The pandemic of coronavirus disease (COVID-19) has been spreading 2019 rapidly worldwide by public transportation, posing a formidable threat to global public health. According to the World Health Organization (WHO), COVID-19 has affected over 200 countries and regions with more than 19.46 million confirmed cases by August 9th, 2020 [7].
One mode of transmission is by droplets as they are conductive for human-to-human transmission of infectious agents. Viral pathogens are spread by droplets expelled by coughs and sneezes, which however can also contaminate surfaces of public transport vehicles. A second not less important problem therefore are contaminated surfaces. Ample evidence in the literature indicates that pathogenic human coronavirus contaminate the environment in a distance of 2m to 6m after sneezing and a single droplet may easily contain an infectious dose [8]. In a simulation experiment, researchers found that aerosols produced by human coughing were easily spread in the entire airplane cabin in 20 seconds [9,10]. Reports of a human lung cell culture model show persistence of infectious viral particles at least 3 days on a range of common non biocidal surface materials, including polytetrafluoroethylene (Teflon; PTFE), polyvinyl chloride (PVC), melamine resin coated surfaces, ceramic tiles, glass, silicone rubber and stainless steel. Contagious viral pathogens are surviving on a surface even longer if epithelial cell material is contained in the droplets. This can easily extend to 5 days [11].
Due to its continuous spread worldwide, long-term effective prevention and control measures should be adopted for different settings and vulnerable groups during this pandemic. Influenza transmission in airport terminals was investigated by modelling studies, which showed the potential for transmission to occur to large numbers of passengers. Accumulating evidence suggests that public transportation, especially air traffic for international travel, contributed not only to the global spread of COVID 19 but also to the dissemination of multi-resistant microorganisms worldwide [12]. The spread of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae during and after international travel is a wellrecognized worrisome problem. The flow of international travellers departing from Taiwan was associated with the carriage of NDM-1- harboring Enterobacteriaceae [13].
The appreciation of the risk of introducing pathogens to new areas could reduce the number of secondary cases at the distant loci who are epidemiologically linked to travellers [14]. In addition pathogens on surfaces occupied by passengers must be cleaned thoroughly after disembarkation. This should be considered for long-distance rail passengers but even more important for air passengers – however it is frequently neglected. For general influenza, a study in Nottingham reported that the chance of developing influenza increased by six-fold for people traveling by public transport within five days of onset of symptoms. Within this period of time of symptom onset people generally do not develop flu symptoms but remain contagious, increasing the infection risk for influenza [15]. Studies also reported that COVID-19 occurred during bus travel [16]. For SARS-CoV-2, recent findings showed also a strong and significant association between COVID-19 and travel by trains [17]. Viral pathogens are surviving on a surface if epithelial cell material is contained in the droplets for an extended period of time. This should be considered for long-distance rail passengers in addition to air passengers. For SARS-CoV-2, recent findings showed a strong and significant association between COVID-19 acquisition and travel by airplane and train. Passengers in airplane cabin had high risks of airborne infectious diseases because the transmission of microorganisms in a cabin was influenced by exhaust ventilation, turbulent diffusion, and advective velocity [18]. The confined aircraft cabin exposes travellers to hypobaric hypoxia, dry humidity, and close proximity to others passengers. Most commercial aircrafts in service recirculate 50% of the air delivered to the passenger cabin. The structural characteristics of airplanes and the mode of mixed ventilation increased the chances of viral spread in the cabin [19]. It is therefore mandatory also to endow air filters with anti-COVID 19 activity.
There was a significant and positive association between the frequency of flights, trains, and buses from Wuhan and the daily as well as the cumulative numbers of COVID-19 cases in other cities with progressively increased correlations for trains and buses (all P values <0.001). The distance between Wuhan and other cities was inversely associated with the numbers of COVID-19 cases in these cities (all P values < 0.001), and the correlation became increasingly stronger and went stable after February 1st. The mean incubation period was estimated to be 5.2 days, therefore real associations appeared after most infected cases out from Wuhan presented typical symptoms and were diagnosed positive. Air transportation appears important in accelerating and amplifying influenza propagation. Transmission occurs aboard aeroplanes, at the destination and possibly at airports. The recommendations are mandatory masking on board, minimizing unmasked time while eating, frequent hand sanitizing, disinfecting high touched surfaces, promoting distancing while boarding and deplaning, limiting onboard passenger movement, implementing effective pre flight screening measures and enhancing contact tracing capability. Control measures to prevent influenza transmission on buses and cruise ships are required too [20-25]. These measures are however not really sufficient to prevent COVID 19 transmission. Disinfection of surfaces after disembarkation of passengers is not feasible and can increase the rate of resistant microorganisms. Besides disinfectants are inactive against microorganisms in a biofilm which occurs regularly on composite surfaces.
A study exploring the transmissions of COVID-19 on cruise lines disclosed also a high impact of COVID 21 transmission. Investigations of COVID transmission in a cruise ship i.e. Diamond Princess estimated that the mean reproduction number (R0) in the confined setting reached values up to 11, which was much higher than the mean estimated from community-level transmission dynamics in China [26]. Taken together, these cases indicated that public transport and passenger terminal stations were likely to become vectors of the virus during the pandemic of COVID-19, resulting in the occurrence of clustered infection or a “superspreading” event. There is evidence that imported cases via public transportation played an important role in the spread of COVID-19. The connectivity and distance between the epicenter and the destination are important determinants of transmission risks. Strong preventive measures have been instituted by 3 g requirements and should also be taken in account in cities with shorter distances and more frequent public transportation in order to contain the COVID-19 epidemic. Although this approach seems to provide sufficient safety, recent experience demonstrated loopholes enabling the recent omicron variant of COVID-19 to distribute worldwide within a few days. This requires innovative and ambitious solutions as demanded by WHO [27]. During the last few years several mechanisms of bacterial resistance have been elucidated, and new insights into the genetic basis of multi-resistance have been gained. Enthusiasm about newly developed antimicrobial agents and disappointment because of the development of resistance have been alternating in the decades since the introduction of antibacterial chemotherapy around 1940. The clinical implications of multi-resistance depend on timely recognition of the problem, i.e. knowledge of the epidemiology of multi-resistant microorganisms and the availability of alternative drugs [28,29].
Areas which are frequented by numerous persons from different countries show also a high bacterial burden. People are confronted with numerous new microorganisms, bacteria and virus with an insufficient resistance profile to many antibiotics. It is particularly complicating if people from different parts of the world are accumulating: In certain areas people are confronted with microorganisms with an unusual high resistance potential (India, South East Asia) people from these parts of the world are not only contaminated with new and unusual microorganisms e.g. COVID 19, bird flu, swine flu but also multiple resistant bacterial pathogens which are distributed in our environment [30]. The global distribution of multi-resistant microorganisms occurs by public transportation in airplanes, cruise ships, trains, buses, trolleys but also in cars (Taxi, car sharing) which are generally not well cleaned after use. Also airports, trains station i.e. the side rails of escalators are heavily contaminated with a variety of multiple resistant microorganisms and are a common hot spot for the distribution of these multi resistant microorganisms [31]. The lack of defense mechanisms e.g. specific antibodies, T lymphocytes against these new microorganisms does not allow immediate elimination of these microorganisms. Genuine self-sanitizing surfaces are however able to prevent the worldwide spread of resistant microorganisms [32].
During the last few years several mechanisms of bacterial resistance have been elucidated, and new insights into the genetic basis of multi-resistance have been gained. Enthusiasm about newly developed antimicrobial agents and disappointment because of the development of resistance have been alternating in the decades since the introduction of antibacterial chemotherapy around 1940. The clinical implications of multi-resistance depend on timely recognition of the problem, i.e. knowledge of the epidemiology of multi-resistant microorganisms and the availability of alternative modes of therapy and prevention. Bacteria and other microbes evolve in response to their environment and inevitably develop mechanisms to resist being killed by antibiotics and disinfectants. With the rapid rise in cases of multidrug-resistant (MDR) bacteria around the world, we are on the verge of entering the postantibiotic era unless something is done immediately to address this global crisis. UN Interagency Coordination Group on Antimicrobial Resistant microorganisms demands immediate, ambitious, innovative measures [33,34]. A number of reasons have been propagated by John Walker [35].
i. Unnecessary prescription of antibiotics.
ii. Inadequate hygiene in hospitals a major factor.
iii. Inadequate hand hygiene Inadequate sanitation in developing countries.
iv. Administration of antibiotics in livestock production. v. Lack of new antibiotics.
vi. Global distribution of resistant microorganisms
However, these reasons – although important - contribute only modestly to the dramatic increased emergence of resistant microorganisms. The emergence of multi resistant microorganisms however developed into a dramatic situation. Millions of patients are affected annually by healthcare-associated infections (HCAI), impacting up to 8 000 patients in European Hospitals on any given day [36]. The Centers for Disease Control (CDC) estimates that 2 million U.S. patients per year acquire a hospital-related infection. These infections cause 90,000 deaths each year. This represents not only a public health risk, but also an economic burden with an average cost of $47,000 per patient to treat. The added cost to hospitals is $ 4.8 billion annually for extended care treatment [37]. Bacteria and other microbes evolve in response to their environment and inevitably develop mechanisms to resist being killed by antibiotics and disinfectants [38-44]. Biofilm formation and subinhibitory concentrations of disinfectants contribute to this phenomenon. Microorganisms have a tendency towards formation of biofilms on plastic surfaces. Microorganisms in a biofilm cannot be eradicated by antibiotics nor disinfectants. At the same time this is difficult to diagnose as microorganisms in biofilms require special culture methods – viable but not culturable (VBNC) [45-47]. Antibiotics and disinfectants are inactive against microorganisms in a biofilm due to hibernation i.e. microorganisms don’t take up anything from the environment e.g. antimicrobial substances as well as disinfectants [48]. The reason: microorganisms in a biofilm are hibernating i.e. in a dormant state of growth and don’t take up anything from the outside. This means that microorganisms in a biofilm are resistant against all antimicrobial agents i.e. antibiotics but also disinfectants.
The most relevant mode of induction of multiresistant microorganisms is the use of disinfectants, which is however not on the scope of the public. There is a law by nature that all substances which have to be incorporated into a microorganism induce resistance. More than 8000 publications in the international literature describe the resistance of bacterial microorganisms against disinfectants and in addition 774 publications describe the cross resistance with antibiotics. At the same time 10513 publications describe the toxicity of disinfectants (April 10, 2022). Contaminated surfaces contribute substantially to the spread of multi resistant microorganisms worldwide [49]. Genuine self sanitizing surfaces with innovative technologies can prevent the worldwide spread of resistant microorganisms [50]. Disinfection of surfaces with disinfectants is not constructive any more as ample evidence exists for tolerance of microorganisms to sublethal levels of various disinfectants e.g quaternary ammonium compounds (QAC) i.e. benzalkonium chloride as well as chlorhexidine, hexadecyl pyridinium and cetrimide [51]. The resistance of QAC based disinfectants to antibiotics is conferred by the resistance determinants qacH and bcrABC. The presence and distribution of these genes have been anticipated to assume a role in the survival and growth of various microorganisms. It has been described that disinfectants (e.g. benzalkonium chloride) even enhance the growth of Listeria monocytogenes in the food industry [52,53].
The use of disinfectants - ostensibly intended to remove/ kill pathogens on surfaces which are ubiquitously contaminated with microorganisms - is not sustainable. Within a short period of time the surfaces can be recontaminated again. The aim of the publication is the documentation of an innovative technology which provides fast and broad antimicrobial activity of surfaces in areas occupied by numerous people and to increase the awareness of other methods to stop the dramatic emergence and distribution of multi-resistant microorganisms. Considering the above information it was mandatory to reassess preventive measures and to curb the dramatic increasing rate of emerging resistant microorganisms. This information is applicable to all surfaces. Antimicrobial coatings hold promise based, in essence, on the application of materials and chemicals with persistent bactericidal or -static properties onto surfaces including textiles/leather used in the healthcare environments but also in public transportation. This belief is based on some preliminary studies involving, for example copper and silver ions, titanium or organosilane, albeit under laboratory conditions. There are however several shortcomings of these technologies.
The development of self-sanitizing surfaces with a broad spectrum of activity, long lasting to permanent antimicrobial activity including multiresistant microorganisms and COVID 19 without induction of resistance seems to be a promising solution for all these problem if the requirements defined for the prevention of hospital acquired infections are met [54-56]. However it is not only prevention of viral pathogens. The presence of blaNDM-5 in K. pneumoniae isolates in China suggests that this pathogen is a potential reservoir of carbapenem resistance genes and could emerge as an upcoming problem [57]. Although this is limited in scope, it shows that public transportation is a potential source of CRE. IncR plasmids carrying multiple resistance genes have been reported increasingly in Enterobacteriaceae isolates. Although these plasmids are known to be non-transferable and nonmobilised, their role in the transmission of resistance has been attributed to transposition events or plasmid recombination leading to multi-replicons, contributing to the high plasticity observed in bacterial plasmids. [58] The presence of carbapenem-resistant Enterobacteriaceae in a public transportation environment is of great concern and indicates that regular antimicrobial resistance surveillance is urgent and necessary. Thus, public transportation agencies should be concerned about the transmission of antimicrobial-resistant bacteria because of the risks they pose to public health. The WHO (Interagency Coordination Group on Antimicrobial Resistance) demands immediate, ambitious and innovative solutions to combat the catastrophic emergence of multi resistant microorganisms [27]. The heavy flow of passengers creates an ideal environment for the transmission of antimicrobialresistant bacteria.
Present solutions i.e. spraying of disinfectants during turn around time of airplanes is by no means sufficient. The careless use of disinfectants is considered the main reason for the catastrophic increase of multi-resistant microorganism. We can´t rely on the efficacy of disinfectants any more. A number of technologies have been proposed with varying applicability.
1) Isopropyl alcohol and ethyl alcohol have been used as lowlevel disinfectants in healthcare settings for many years. Recent studies have shown that ethyl alcohol inhibits protein synthesis in Escherichia coli by direct effects on ribosomes and RNA polymerase and that 60%-70% solutions have in vitro efficacy against murine norovirus, Ebola virus, and several coronaviruses.
There is a concern regarding the rapid evaporation rate of alcohol and hence the associated short duration of antimicrobial activity. Alcohol is suitable therefore only as alcohol prep pads or towelettes containing isopropyl or ethyl alcohol and water for primarily use for disinfection of small noncritical items. After strong emphasis has been put on increased hand disinfection with alcohol a vancomycin resistant enterococcus has been isolated in 230 patients in a Swiss hospital. This germ is alcohol insensitive! [59] For disinfecting environmental surfaces in healthcare facilities it has been recommended to combine ethyl alcohol and/or isopropyl alcohol with other active agents such as quaternary ammonium or phenolic compounds. However the use of disinfectants is associated with rapid emergence of multiple resistant microorganisms against disinfectants and cross resistance with antibiotics. More than 9700 publications on resistance disinfectants are available in Pubmed (International literature survey) and also 10,800 publications on “Toxicity Disinfectants“ are available.
Additional substances have been investigated for activity against COVID 19: Active drug eluting agents e.g. ions or (nano)particles of silver, copper, zinc, chloride, iodine have been propagated [60-64]. Silver has been in use since years. Free silver ions must be incorporated into the metabolism of microorganisms: therefore silver must be eluted from a surface. This means that the activity of silver is limited to 1 – 3 weeks as silver nanoparticles are depleted in the surface. If used as nanoparticles additional problems arise: The antibacterial mechanisms of NPs are poorly understood, but the currently accepted mechanisms include oxidative stress induction, metal ion release, and non-oxidative mechanisms. However nanoparticles cannot be incorporated into polymers, the fixation on a surface in coatings as nanorods or nanomats proved to be difficult and was not sustaining. It has to be approved by nano legislation which is time consuming and expensive. The release of Ag(i) from silver nanoparticles (AgNPs) unintentionally spread in the environment is suspected to impair some key biological functions [65]. Copper endowment of a surface has been investigated and preliminary results show a moderate reduction in the number of HAI as well as COVID 19 Cu has the potent capacity of contact killing of several viruses, including SARS-CoV-2. Several adverse events have been observed. Copper shows
i. A limited antimicrobial activity against bacterial pathogens. This activity is somewhat improved by alloys e.g. combination with zinc or tin but still does not meet the full requirements.
ii. The surfaces are rapidly oxidized and require continuous cleaning.
iii. Copper cannot be used in cable insulations.
iv. Copper cannot be used as transparent coatings of surfaces.
v. The majority of surfaces are preferably white or transparent, the color of copper is not accepted by the majority of people
2) Additional antimicrobial substances have been propagated [66]. Chlorine containing disinfectants, UV irradiation, hydrogen peroxide and surfactants. Among these groups, bleach (chlorine containing disinfectants) have frequently applied. Furthermore hydrogen peroxide, povidone-iodine, chlorhexidine and UV irradiation has been used. All of them are associated with substantial disadvantages and toxicity [67]. Also other substances e.g. Polybiguanides, halogenated phenols, and polyethyleneimines have investigated for activity against COVID 19 in different environments. While sufficient activity has been demonstrated: PHMB-treated fabrics kill 99% of the coronavirus within 2h. PHMB was associated with severe/lethal pulmonary toxicity preventing the widespread use [68]. Polyguanides and halogenated phenols did not exhibit sufficient anti- COVID activity. Halogenated Phenols (HP) are responsible for endocrine and neuronal persistent organic pollutant effects, suspected carcinogenic [69]. Dichlorinated parabens accumulate in solution, which is a threat to human health and the aqueous environment [70].
3) Antimicrobial Peptides: The application of antimicrobial peptides provide an interesting concept i.e. by copying the body’s defense mechanisms. AMPs are amphophilic acid peptides with 36- 38 amino acids, produced by epithelial cells upon contact with a pathogen and show broad antimicrobial activity. The antimicrobial activity of AMPs is based on disruption of the cell wall of pathogens, which leads to a fast eradication of microorganisms [71]. AMPs have to be attached to polymer brushes for application on surfaces: Polymer brushes are necessary as antimicrobial molecules lose much of their activity once attached to a surface. When providing an anchor for the active molecule through a flexible covalently bound polymeric chain, the active molecule should still be able to reach the site of action at or within the bacterium, e.g. by penetrating its cell wall, but leaching is still suppressed. AMPs have to be eluted from a surfaces and interact directly with pathogens. This means that the antimicrobial activity is limited to a few days. Important parameters for polymer brush anchors are chain length and chain density. Polymer brushes have been shown to be effective for anchoring AMPs but also QACs. However substantial problems arise with the use of AMPs
a) AMPs have to be eluted from the surface and must directly attack microorganisms on their surface –Therefore the activity is limited to a few days.
b) AMPs are not heat stabile and cannot be incorporated into polymers
c) AMPs are not easy available in large quantities. They could be obtained as Magainins from the skin of the African frog Xenopus laevis with very limited availability [72].
d) Synthesis of AMPs is not solved as AMPs are lethal factors for microorganisms.
Investigations over 2 years disclosed synthetic AMPs induce fast resistance against microorganisms-these microorganisms are in turn also insensitive to natural AMPs produced by the body. Definitely to be avoided! [73].
4) UV light has been recommended for eradication of COVID 19 on surfaces [74]. There is little scientific information on the activity of UV light against COVID 19. UV light however has substantial side effects e.g. carcinogenicity, eye irritation and damage to the skin as it accelerates the aging of skin by damaging the collagen fibres under it. UV light is therefore not suitable neither alone or in combination with silver or disinfectants in airplane cabins to eradicate COVID 19 on surfaces. A combination with e.g. silver nanoparticles has been recommended with limited applicability [75].
5) Anti-Adhesive Surfaces: Anti-adhesive surfaces have been designed to reduce the adhesion force between bacteria and a solid surface to enable the easy removal of bacteria before a biofilm layer is formed on the surface. Such surfaces may suppress HCAI by blocking transmission paths on surfaces, but they will not reduce the number of germs on the contacting media by killing them. Attachment of bacteria or cells starts with an initial adsorption of proteins on to the material surface. Strategies to prevent protein attachment include superhydrophobic surfaces, often augmented by a hierarchical nanostructure as well as zwitterionic polymers [76]. One of the most important requirements of “selfsanitizing” surfaces is the ability of the surface to actively eradicate pathogens within a short period of time. It has to be emphasized that microorganisms are deposited by the hand of the personnel with considerable force. For prevention of recontamination of a second person, rapid eradication of microorganisms (i.e. less than 1 hour, preferably less than 30 minutes) is mandatory. Reduction of adherence, blockage of proliferation and biofilm formation – although important – is in no way sufficient as “self-sanitizing” surfaces for clinical use or in public transportation to prevent recontamination of persons. The technology provided in this publication is able to eradicate microorganisms deposited by hands within 30 minutes. The antimicrobial activity has been determined on a surfaces endowed with in situ generated biocides by the push plate (RODAC plate method). Initial inoculum sizes is 109CFU/ml, Melamin resin 2 % Zinc Molybdate (Figure 3).
6) Contact-Active Surfaces: Contact-active surfaces exhibit antimicrobial activity without releasing biocidal substances. Several mechanisms are believed to take place in contact-active surfaces. These are:
i. A so-called spacer effect, where the biocidal group is attached to the surface through a polymer chain, allowing the biocide to reach the cytoplasmic membrane of the bacteria and to perforate them;
ii. Alternatively, positively charged QACs, e.g. 3-aminopropyl trimethoxysilane grafted to cellulose nanofibres, can detach phospholipids from the cell membrane and thereby kill the bacteria [77]. This approach is also referred to as biomimetic with respect to the activity of chitosan a polysaccharide derived from exoskeleton of crustaceans or cell walls of fungi. Hydrophobic parts of a surface can act similarly to QACs by deforming the membrane through adhesion [78]. The activity of the spacer effect is obliterated by grease, proteins, sweat, pus and blood. The activity of chitosan has been investigated and a poor antimicrobial activity has been found. In regards to Nanotechnology and QACs see comment above.
7) Immobilizing bacteriophages on surfaces. An entirely different approach has been described by immobilizing bacteriophages on surfaces. Bacteriophages are viruses that infect and kill bacteria. Bacteriophages are host specific, but they can have a broad host range, infecting several strains or species of bacteria both Gram-positive and Gram-negative. However they show high specific efficiency against relevant bacterium. Different microorganisms require different phages – there is no uniform broad spectrum of activity against all bacteria, fungi, molds and virus - in essence the adequate phage must be selected from more than 10000 different phages. This requires identification of the microorganism responsible for the contamination and selection of the adequate phage prior to the application. Phage therapy is therefore inefficient for the application “self-sanitizing surfaces” as there is no uniformly effective phage with broad spectrum activity for application in surfaces [79,80]. Phages cannot be incorporated into coatings on stainless steel, aluminum, enamel etc. Attachment of bacteriophages to a surface can be achieved through physisorption, electrostatic attachment, and covalent bonding. Besides phages are not heat resistant and are also eluted from the surfaces with limited duration of activity.
8) UV light has been propagated for eradicating microorganisms from a surfaces. The antimicrobial activity of UV light requires additional considerations. It is important to standardize the position, distance and time for UV light application and also to determine its efficacy against medically important bacteria, bacterial spores and fungi. Besides there are serious adverse events which question the clinical usefulness of this technology. The energy of UV light is sufficient to induce mutations in DNA. Studies show that UV rays damage DNA, cause gene mutations leading to the development of malignant tumors. UV light is considered carcinogenic to the skin. Ocular tissues are also exquisitely vulnerable to ultraviolet light and prevent the continuous application [81].
9) In Situ Generated Biocides: A completely new approach for antimicrobial activity has been investigated. The antimicrobial activity of the concept of Brønsted-Lowry Lewis acids with MoO3 or the 5% oxygen deficient Tungsten blue oxide has been investigated [82]. Among the various metal oxide semiconductors molybdenum trioxide attracted attention due to its multifaceted functional properties and its application as catalyst in various selected oxidation reactions, sensors and photochromic and electrochromic systems. In this manner, MoO3 and WO3 are interesting electrochromic inorganic materials. WO3 film exhibits a broad range of functional properties, such as small band gap energy (2.4-2.8eV), deeper valence band (+3.1eV), stable physicochemical properties, and strong photo-corrosion stability in aqueous solution. Transition metal oxides are eager electron donors: electron transfer occurs to ambient water and results in the formation of various substances for fast and efficient eradication of microorganisms from surfaces..
a) The antimicrobial effect of acids is well known. Hence the idea was to use acid surfaces for antimicrobial properties imitating the body´s own defense mechanisms i.e. acid coating of the skin [83]. Formation of an acid surface by formation of H+ ions from ambient water has been documented. The surface pH can be determined by a surface pH electrode (Sentex) A pH between 4.6 and 4.2 is regularly achieved by transition metal oxides incorporated into a polymer or a coatings on the surface of samples. This corresponds to the pH of the acid coating of the skin.
b) In addition to the formation of acidified water molecules free radicals e.g. oxygen radicals, hydroxyl radicals are formed [84]. The photooxidation of water to oxygen and protons in the presence of reducible additives of molybdenum oxide and tungsten oxide has demonstrated the photocatalytic activity of the substances. Hydratide Oxonium-Ions, (H3O) (OH2)n (n=1,3) strip their hydrate water in contact with microorganisms, finally the water molecule. The naked protons penetrate the phospholipid bilayer of bacterial microorganisms by denaturation of the protein envelope. Protons inhibit the activity of vital enzyme systems within the microorganisms which require a narrow pH environment. This is called protolysis (Coagulation necrosis).
c) A positive Zeta potential has been observed. An electro positive charge is initiated at the surface which attracts electronegative charged microorganisms. This results in an almost immediate discharge at the surface and a disruption of the phospholipid bilayer of microorganisms. This is the reason for the rapid bactericidal activity of surfaces which has been documented by laser scanning microscopy [85-87]. Within less than 15 minutes 109 colony forming microorganisms on a 3cm² cm surface are eradicated. This is also observed for microorganisms contained in a biofilm [88].
d) Paramagnetic Ions, documented by EPR spectra have the least clear described antimicrobial activity but contribute to the synergistic activity of the above described technologies [89]. As the antimicrobial products don’t have to be incorporated into the metabolism of microorganisms no induction of resistance in contrast to disinfectants has been observed! The technology is therefore also active against microorganisms in a biofilm.
e) There is a Synergy of Antimicrobial Activity by all these Mechanisms: Protons also destroy the fimbria preventing adherence of germs on surfaces [90]. The characteristics of WO3 film make them suitable for electrochromic layers e.g. in a smart window. Many studies pertaining to WO3 structures are mainly aimed at the formation of high active surface charge in view of their use in electrochromic applications [91]. Additional investigations disclosed that he incorporation of molybdenum oxide into the Zinc oxide (ZnMoO4) crystals are also strong semiconductor inorganic solids and show extensive electronic properties in various scientific fields [92,93]. Important for the electrochromic property is a crystal structure which is monoclinic and orthorhombic.
All these electrochemical properties/high energy potentials have been found to disrupt the phospholipid bilayer of microorganisms bacteria fungi and virus by electron transfer and high voltage surface charges documented by a positive zeta potential and electro paramagnetic properties documented by a high number of electro spins [92]. The voltage charges are confined to <5μm on a surface. This suggested that besides the H3O+ there exist additional mechanisms of antimicrobial activity like paramagnetic Mo5-Ions. Results of electro paramagnetic resonance (EPR) – spectroscopy supports this theory. From 77 K registered spins per Gram the molar concentration of paramagnetic Mo5- Ions was calculated. One crucial property for the electron transfer is a hydrophilic surface with a contact angle of 30° or less. This can be achieved by numerous hydrophilising agents e.g. glyzerine stearate, Sorbitol Ester, Crodamol, Crodaphos, Fleroxacin and Lubrophos. Additional hydrophilising agents e.g. are Tween 20 and 28 have been investigated with excellent hydrophilicity of the surfaces.
MoO3 has a water solubility of 0.003mol/l at a pH value > 7.55, which is even lower when it is incorporated into polymers. Tungsten and its sub oxides are water insoluble. Investigation of reduction of the water solubility of molybdenum oxide resulted in completely new compound. The incorporation of molybdenum oxide into the tungsten crystal lattice in various concentrations renders also Molybdenum completely water insoluble [94]. Elution experiments of polyoxometallates (POMs) in a 100cm² surface for 7 days in 1 l deionized water disclosed >0.0002mg/l of Mo oxide and for Tungsten oxide below the level of detection. This also results in a long lasting antimicrobial activity. No toxicity was detected in extensive investigations. Paramolybdotungstate with the formula [H2Mo6W6O42]10 can be produced as the precursors assemble by themselves in the same crystal structure due to their similar ion radius. The incorporation of Molybdenum oxide into the tungsten blue crystal lattice results in a new product albeit under the patent protected technology of in situ generated biocides. Compounds containing Mo oxides and W oxides in various concentrations in the same crystal lattice and are considered a new compound called polyoxometallates [POM] with additional properties. The 5% oxygen deficient Tungsten blue oxide and polyoxometallates MoxW1-xO3 show similar antibacterial activity compared to Molybdenum oxide and Zinc Molybdate [95]. Various additives show specific properties and can be applied accordingly for various different purposes. There is antimicrobial activity against all pathogens covered by Molybdenum oxide, Tungsten blue oxide, POM and Zinc Molybdate. A number of additional properties have been observed. POMs exhibit strong antimicrobial activity against virtually all bacterial microorganisms regardless of their resistance to antibiotics and disinfectants in addition to fungi, molds (Aspergillus spp.), enveloped and nonenveloped virus based on the strong Zeta potential. Polyoxometallates show also high activity against microorganisms embedded in biofilms. The crucial pathogenic role of free radical damage in respiratory virus induced pneumonia suggest an antioxidative strategy for COVID-19. The potential anti-SARS activity (severe acute respiratory syndrome) of the POMs [alpha-PTi(2)W(10) O(40)](7-) isomers has already been suggested by Hu [96]. The SARS 3c-like protease, namely SARS 3CL(pro), is the key function of the protease for both viral replication and transcription and can therefore be considered as one of the main targets for the development of anti-SARS drugs. Polyoxometallates incorporated into surfaces however show a substantial increased antiviral activity compared to Titanium oxide [97].
For Public transportation this means that all surfaces in airplanes, trains and trolleys but also in buses, cars (taxi, car sharing) could be endowed with submicron polyoxometallates in a concentration of 1-2 %. This can be achieved by embedding POMs in paints, polymer surfaces, textiles, leather and artificial leather. In airplanes also the air filters have to endowed with anti SARS polyoxometallate activity. Also freezing on various surfaces is prevented by transition metal oxides incorporated into polymers. Last not least also a strong antifouling activity has been documented. All these electrochemical properties/high energy potentials have been found to disrupt the phospholipid bilayer of microorganisms bacteria fungi and virus by electron transfer and high voltage surface charges documented by a positive zeta potential and electro paramagnetic properties documented by a high number of electro spins. The voltage charges are confined to <5μm on a surface. This suggested that besides the H3O+ there exist additional mechanisms of antimicrobial activity like paramagnetic Mo5-Ions. Results of electro paramagnetic resonance (EPR) – spectroscopy supports this theory. From 77K registered spins per Gram the molar concentration of paramagnetic Mo5- Ions was calculated.
Since the antimicrobial mechanism of in situ generated biocides is non-specific, there is a broad spectrum of activity including Gram positive and Gram negative microorganisms irrespective of their resistance against antibiotics, spores, fungi, Aspergillus spp., Legionella, viral organisms e.g. influenza (H1N1, H5N1) including enveloped viral pathogens, Norovirus [98-100]. As the activity is not based on incorporation into the metabolism of microorganisms, bacteria don´t develop resistance against this mechanism as is the case with antibiotics and organic biocides. In this work, 2% MoO3 was mixed into the thermoplastic polymers TPU, PP and PVC, liquid silicone and epoxy resin. It has to be emphasized that this technology is dependent on a hydrophilic surface. This can be achieved by a number of hydrophilising agents e.g. Glyzerin Stearate 1% (Atmer) or other compounds like Crodamol, Fleroxacin, Lubropshos again in a 1 % concentration. For different polymers certain hydrophilising agents are favored and must be investigated accordingly. A contact angle of 30° or less is mandatory for the antimicrobial activity. The polymer samples were obtained on an extruder (Berstorff ZE25A), and the polymer was shaped into 10 x 10cm² plates using a heated press. The epoxy resin was a two-component system consisting of 60 parts resin and 40 parts hardener. Silicone samples were obtained . The microbiological tests were carried out with 109CFU/ml (CFU = colony forming units) of three reference bacteria: Staphylococcus aureus ATCC 25923 (S.a., a typical germ on human skin, Grampositive) Streptococcus faecium: alcohol insensitive clinical isolate. Escherichia coli ATCC 25922 (a typical germ in excrements and a lead indicator for fecal contamination, Gram-negative) and served as prototype for numerous other Gram negative microorganisms
Pseudomonas aeruginosa ATCC 15442 (Pa. a typical germ in air, soil and water). Carbapenem resistant Klebsiella spp. just as numerous other multiresistant Gram negative microorganisms (e.g. Acinetobacter Baumannii, Serratia marcescens) are also covered by the spectrum of activity. Similar experiments have been performed with 180 fresh clinical isolates isolated from hospital acquired infections. The determination of anti-microbial effectiveness was done by the push plate method and the roll-out method, which are cost-effective and semi-quantitative tests resembling the “real life situation”. Also Legionella spp, Candida spp, are included in the spectrum of activity. Polyoxometallates show also strong activity against Aspergillus spp. like A. fumigatus and A. niger. Activity of transition metal oxides (Polyoxometallate) against a number of viral pathogens has been demonstrated (bird flu, swine flu, Influenza, Herpes virus, Respiratory syncytial virus, Epstein Barr Virus, EBV) [101]. The activity against COVID is documented by MSL laboratories, UK [102]. Various Products of Transition Metal Oxides are Available With Similar Antimicrobial Activity and are applied according to the characteristic properties.
Molybdenum trioxide is available as a light blue/gray powder with particle size of 2-5μm.
• Advantage: Strong antimicrobial activity with addition of 2 % to various polymers e.g. TPU for ECG lead wires which has been documented in numerous experiments and clinical application. Thermal induced fracturing of the hydrates of molybdenum trioxide retains the orthorhombic crystal structure with particle sizes of 0.2μm. (Lambda half) The incorporation in various coatings e.g. liquid silicone, liquid polyurethane results in a antimicrobial activity on glass and stainless steel. Acid surfaces formed by Brønsted- Lowry acids also prevent effectively adherence of microorganisms on surfaces, block proliferation and biofilm formation. The powder is inexpensive and available in unlimited quantities. Safety and side effects of transition metal oxides have been investigated. The nasal respiratory epithelium in male rats exposed to 30 or 100 mg/m3 has been investigated.
i. Causes eye irritation.
ii. May cause respiratory irritation.
iii. The incidences of hyaline degeneration in and in all exposed groups of females rats were significantly greater than those of the control groups. Incidences of hyaline degeneration in the nasal olfactory epithelium of all exposed groups of females were also statistically significant. For male mice, the incidences of histiocyte cellular infiltration in all exposed groups were significant. Incidences of hyaline degeneration of the respiratory epithelium of the nasal cavity in female mice at 100mg/m3 were significantly greater than those in the controls (NTP 1997). Based on the 2-yr NTP study, the LOAEL is 10mg/ m3 for increased incidences of hyaline degeneration in the nasal respiratory epithelium and nasal olfactory epithelium in female rats. Precautionary statement: Wear protective gloves/ protective clothing/ eye protection/ face protection. Molybdenum trioxide is not UV light stabile.
The safety data sheet describes carcinogenicity. Two 13-wk studies were conducted by the national toxicology program (NTP, 1997) in which F-344/N rats and B6C3F1 mice (10/ sex/group) were exposed to molybdenum oxide by inhalation of submicron particles at concentrations of 0, 1, 3, 10, 30, or 100mg/m3 for 6.5hr/d, 5 d/week for 2 years. All rats and mice survived to the end of the study, however pulmonary malignancy has been observed in 50 % of rats, no mice were affected by pulmonary malignancy with the same study protocol. Molybdenum oxide shows water solubility of 0.003mol/l at a pH value > 7.45. Elution experiments of a 100cm² TPU surface containing 2 % MoO3 for 7 days in 1 l of deionized water disclosed a concentration of 0.0002mg/l of Mo, the Zinc Molybdate and Tungsten blue oxide concentration applied in a concentration of 2 % was below the level of detection. In the rare case the Zinc Molybdate is eluted from a polymer or a coating, Molybdenum and not molybdenum oxide is eluted. Molybdenum itself does not show carcinogenicity. Molybdenum oxides as well as tungsten blue oxide, incorporated into composite materials e.g. TPU, PE, PP or various coatings like liquid silicone, melamine resin various lacquers are not available as submicron particles for inhalation. Therefore this study regarding carcinogenicity of transition metal oxides is of no relevance for in situ generated biocides embedded in polymers or coatings. However for masterbatch production this information is of relevance. A dust-free application of Molybdenum oxides into composite materials for masterbatch production is required but this is considered state of the art. Similar results have been suggested for Zinc Molybdate with identical lack of relevance in polymers and coatings.
Significant increases in liver copper concentrations were observed in female mice exposed to 30mg/m3 and in male and female mice exposed to 100mg/m3 oral application (males: 11.51μg/g in the 100mg/m3 exposure group versus 8.19μg/g in controls; females: 6.51 and 6.98μ/g in the 30-and 100mg/ m3 dose groups, respectively, versus 5.68μ/g in controls). The increased copper concentrations were not regarded as being an adverse effect relevant for deriving a LOAEL and a NOAEL. No other clinical findings were observed in either rats or mice. Additionally, no significant differences in absolute or relative organ weights, sperm counts, or motility were noted in rats or mice. In the same NTP (National Toxicology Program,) study 1997, rats (F344/N) and mice (B6C3F1) (50/sex/ dose) exposed for 6hr/d, 5d/wk at concentrations of 0, 10, 30, or 100mg/m3 molybdenum trioxide for 2yr experienced a significant exposure-dependent increase in blood Mo concentrations. Male and female rats exposed to 30 or 100mg/ m3 experienced significantly increased incidences of chronic alveolar inflammation. Elution studies from various polymers and coatings with 2% MoO3 over 7 days showed concentrations below the level of detection. The toxicity of Molybdenum is remarkable low. It has to be emphasized that molybdenum – as well as zinc are essential trace elements in the body. Molybdenum is a stabilising molecule in several enzymes important to animal and plant metabolism for elimination of sulfur from the body [102]:
a) Sulfite oxidase catalyses the oxidation of sulfite to sulfate, necessary for metabolism of sulfur amino acids. Sulfite oxidase deficiency or absence leads to neurological symptoms and early death.
b) Xanthine oxidase catalyses oxidative hydroxylation of purines and pyridines including conversion of hypoxanthine to xanthine and xanthine to uric acid. Aldehyde oxidase oxidises purines, pyrimidines, pteridines and is involved in nicotinic acid metabolism.
c) Low dietary molybdenum leads to low urinary and serum uric acid concentrations and excessive xanthine excretion. d) Molybdenum functions as an electron carrier in those enzymes that catalyse the reduction of nitrogen and nitrate in fertilisation.
Hazard statements for Molybdenum powder in the safety data sheet discloses adverse effects by direct contact:
Cytotoxicity of Molybdenum oxide has been determined using the 3-(4, 5-Dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide MTT or NBT assay. This assay measures the reducing potential of the cell using a colorimetric reaction. Viable cells will reduce the MTS reagent to a colored formazan product.
• Results: No toxicity has been determined Survival of MRC5 (immortalised mouse lung fibroblasts) cell line showed with a concentration of 2 % MoO3 in TPU 97 % survival 2 % MoO3 in Epoxy resin 98 % survival
• Using the NBT/MTT Test: No colorimetric difference between controls and verum has been observed. Vero cells, osteoblasts, fibroblasts are growing on these surfaces
Molybdenum trioxide can be used for an antimicrobial activity of any surface which is not in constant water contact. The light blue color cannot be concealed by additional color pigments. As an in situ generated biocide the Molybdenum oxide is permanent fixed in a polymer or a coating. There is no contact with the microorganisms. Information on the carcinogenicity of calcium and zinc molybdates was not found.
Tungsten Trioxide has been investigated for in situ generated biocidal activity. Initial experiments with the oxygen saturated Tungsten yellow oxide disclosed limited antimicrobial activity. However when there was a mix of tungsten yellow and blue in the samples obtained non conclusive results have been detected.. Further investigations disclosed that the 5% oxygen deficient tungsten blue oxide WO2.75-2.90 shows antimicrobial activity comparable to Molybdenum oxide. Tungsten oxide is a dark blue powder and is available in 5μm particle size. Fractioring with a preserved crystal lattice can result in a transparent coating with 0.25μm particle size
• Advantage: Tungsten blue oxide is completely water insoluble, the safety data sheet shows no adverse reactions except for necessary individual contact measures. Again this is of relevance to the masterbatch producer as tungsten oxide is not released from polymers or coatings. Tungsten blue oxide can be used for surfaces in permanent contact with water e.g. pipes. Tungsten blue oxide is a suitable addition to kautschuk e.g. for siderails in escalators. No carcinogenicity has been described.
The combination of Molybdenum oxide with Zinc oxide in the same crystal lattice in a triclinic orthorhombic crystal structure shows excellent antimicrobial activity plus additional features. This is the preferred compound for in situ generated biocide e.g for endowment of various polymers e.g. melamine resin for hospital furniture, in TPU for ECG wires and liquid silicone where a white color is mandatory. Zinc molybdate is an excellent choice. Zinc molybdate is neither water nor alcohol soluble and stays firmly inside a polymer or paint. It is not toxic and can be used as a corrosion prevention agent, a flame retardant and smoke suppressant compound. Zinc Molybdate is UV light stabile. The strength of the antimicrobial activity is comparable with Molybdenum trioxide with activity against Gram positive and Gram negative microorganisms, legionella, spores and fungi. In addition also influenza virus (bird flu, swine flu) is included in the spectrum of activity. Zinc molybdate is UV light stabile, nontoxic. Zinc molybdate is available in 2μm, 5μm and 8μm particles sizes. Zinc Molybdate can also be synthesized as 0.2μm particles in fluids in unlimited quantities. Zinc Molybdate has a broad spectrum of applications e.g. in paints, textiles, leather artificial leather for public transportation where a white color is mandatory. Flame retardant/ smoke suppressant properties make Zink Molybdate suitable for application in airplanes but also in trains, trolleys, buses and in cars for car sharing and rental vehicles. Zinc Molybdate can also be used for heat exchange equipment and in air filters in air conditioners in airplanes and cars. Most uses are for paints. No carcinogenicity is described. Skin tolerance test have been performed of lacquer samples with Zinc Molybdate incorporated in a concentration of 0.25% (coatings) and 3% in composite materials: skin tolerance tests have been performed in 10 persons with a 5 x 5cm lacquer sample of Zinc Molybdate 3% over a period of 24, 48, 72 and 120 hours on their forearm in a wet chamber. No adverse effects have been seen during the test period. No allergy was observed during one year 1 year following the initial observation.
The observation of a moderate water solubility of Molybdenum trioxide prompted the development of polyoxometallates i.e. incorporation of the Molybdenum trioxide into the tungsten trioxide crystal lattice. Polyoxometallates are available by commercial producers e.g. in unlimited quantities. Polyoxometallates can also be synthesized by the Pharmjet (additional investigative work on the basis of limited preliminary results)
• Advantage: Water insolubility of molybdenum in the combined crystal lattice in Tungsten has been documented. Additional strength of antimicrobial activity against Gram positive and Gram negative microorganisms and spores due to a strong Zeta potential has been established. 109 CFUs. of S. aureus ATCC 25923 and E coli ATCC 25922 are eradicated in less than 30 minutes. Polyoxometallates 2% Mo:W 1:1 have been incorporated into various polymers, textiles (coating with SiO2) leather and gloss paints (Figure 4). Excellent activity against a number of viral pathogens (influenza, hepatitis B, C, Flavivirus, HIV, RSV, RNA Virus, (EBV), Herpes simplex, as well as Norovirus has been detected with Polyoxometallates in polymers or in gloss paints (Airplane tables) 1% Mo:W 1:1. The crucial role of free radical damage of microorganisms in COVID-19. Has been investigated [87]. The test product received has achieved a 2.11 log reduction (99.22%) against Feline coronavirus when tested under the condition stipulated in this report. MSL Gollinrot Walmersley, UK. The substantial advantage of Polyoxometallates is a strong activity against COVID 19. The activity is comprehensive and will work also against various variants already developed and emerging in the future. Additional antimicrobial activity against moulds (Aspergillus spp) Legionella pneumophila has been documented. Strong antifouling activity has been documented including activity against algae. These properties recommend the use of polyoxomtallates as addition to filter systems in airconditioners for application in airplanes in motor vehicles and many more. Several additional applications are possible aside from public traffic and many additional applications.
All the above described additives as powder with particle sizes of 0.25 – 2-8μm can be added to various polymers e.g. TPU, PE PP, HDPE, Polystyrol, Polyimine, silicone. The surface has to be hydrophilic i.e. wettable with a contact angle of 30°. This can be achieved by the addition of various hydrophilising agents (preferably sorbitol alcohol in Glyzerin Stearat and other products e.g. Crodamol, Fleroxacin, Lubrophos in a concentration of 1 %). Smaller particles (0.2μm particles sizes) can be produced by thermal induced fractioring or by synthesis which preserves the orthorhombic and monocline crystal structure required for antimicrobial activity. Submicron particles embedded into transparent coatings are used for transparent antimicrobial activity on glass. Last not least non icing activity of surfaces endowed with Zinc Molybdate 2% has been detected.
It is crucial to investigate how fast microorganisms are eradicated on a surface after hand contact. Investigation of antimicrobial activity investigation has been performed after hand contamination of surfaces close to a real life situation. It has been demonstrated that an assumed contamination with 109CFU/ml (lawn) is eradicated virtually within 30 minutes. Finger imprints have been investigated on an artificial leather seat endowed with Zinc Molybdate 2 % (Figure 5).
a) JIS method (Japanese industrial standard). testing is required by the biocidal product regulation (BPR) of the European Union. This test has been assigned for the investigation of the antimicrobial activity of Titanium oxide. The activity of Titanium oxide is based exclusively on the activity of oxygen radicals As these oxygen radicals evaporate rapidly into the environment, the surfaces is covered with a foil. In this test the number of microorganisms between a surface and a foils is investigated, where unrealistic concentrations of oxygen radicals accumulate. This method is far from the real life situation, therefore the investigations have been performed according to the RODAC plate method. A realistic test method is the RODAC (Replicate Organism Detection and Counting) push plate method used in all our experiments: However also the JIS method if used simultaneously to satisfy the requirements of authorities. Microorganisms (ATCC reference strains) are stored in cryopellets at –25 °C. Prior to the investigations pellets are grown in Isosensitest broth for 6 – 8 hours. In the evening microorganisms are transferred from liquid medium to blood agar plates with the addition of 5 % defibrinated sheep blood at 37 °C. “overnight cultures”. After 12 hours colonies are harvested and suspended in physiologic saline at a final concentration of 109CFU/ml. The number of colony forming units for contamination of the surface is determined by a photometric method: an OD of 0.33 at 475nm reveals a concentration of 109CFU/ml of Staphylococcus aureus. Final concentrations were determined by several 1:10 dilutions until colonies were countable. Identical results are obtained with both methods. Antimicrobial results of paints with the JIS method: 0.25 % Zinc molybdate is incorporated into various paints + 1 % glyzerine stearate (Ganzlin Powder laquers, Mankiewicz gloss paints, Mankiewicz Strukturlack med. blue, Mankiewicz Fenstergrau, Mankiewickz Strukturlack Schiefergrau) compared with two controls.
a) Zinc Molybdate: Zinc Molybdate results in a white or transparent surface if a particle size of 0.25μm (lambda half) has been applied and can be added to virtually every substrate e.g. composite materials, paints, leather, artificial leather, textiles. Zinc Molybdate is water insoluble, no toxicity is described. This additive is also flame retardant and smoke suppressant.
b) Tungsten blue oxide: Due to its complete water insolubility this additive is suitable for endowment of surfaces with permanent water contact e.g. pipes etc.
c) Polyoxometallates: Polyoxometallates i.e. incorporation of molybdenum oxide into the tungsten blue crystal lattice shows besides antibacterial activity, and activity against fungi and moulds strong antiviral properties including COVID 19. In situ generated biocides are insoluble in water, alcohol acids and not are eluted from the surface. No toxicity is observed. Upper limits of intake which also includes children and lactating women are 500 times higher than the allowed concentrations in real life observed. Results of antimicrobial activity testing of gloss paints on airline seats containing 0.25 % of Zinc molybdate by the RODAC push plate method (Figures 6-9).
Figure 7: Investigation of a gloss paint lacquer sample by the RODAC (Replicate Organism Detection and Counting) push plate method.
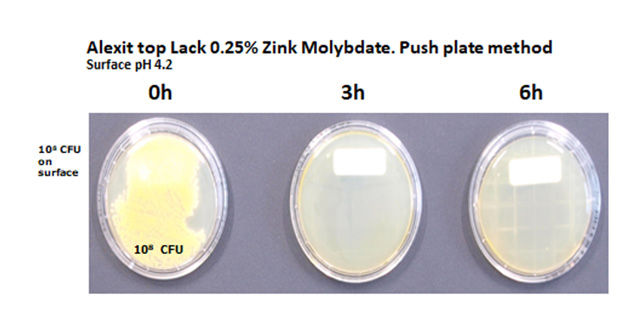
10μl of the bacterial suspension containing 109CFU/ml is applied to a 1 cm² surface of the material under investigation. The drops are dispersed over an area of 1 x 2.5cm with a Drigalski spatula. The liquid dries on the surface within 15 minutes. From the initial inoculum (0 hour) after one hour and in three hourly intervals thereafter until 12 hours RODAC Plates (Caso Agar) are pressed onto the contaminated areas. The push plates are incubated at 37 °C for 24 hours. Then colonies are counted, the results are documented by photography. Results of antimicrobial activity of Zinc Molybdate 2 % in liquid silicone and SiO2 coating against S. aureus, ATCC 25923 push plate method. Samples with 2 % polyoxometallates in TPU and PP show 3 years after the initial investigation identical results. The spectrum of activity of polyoxometallates also contains a number of virus e.g. Hepatitis B, Hepatitis C, RNA Virus, Herpesvirus and HIV. Investigations of samples containing 1 % Polyoxometallates performed by MSL Laboratories disclosed a 99.22 % reduction of coronavirus surrogate virus by polyoxometallates (Figures 10 & 11).
Figure 10: Zinc Molybdate 2% in various coatings shows a reduction of the original inoculum size of 8 log 10 of S. aureus ATCC 25923 of airplane seats within less than 3 hours.
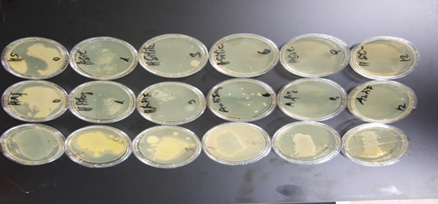
Figure 11: Polyoxometallates Mo: W in a ratio of 1:1 incorporated in a concentration of 2 % in airplane tray tables.
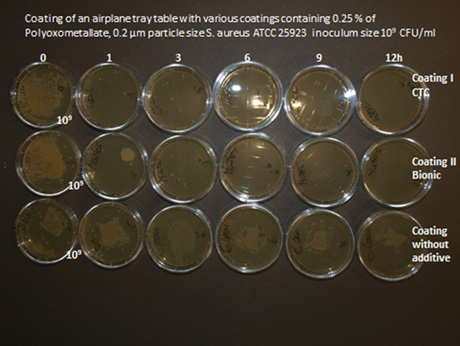
Transition metal oxides can be incorporated into various polymers and coatings e.g. liquid silicone, liquid polyurethane, silicium dioxide. It is essential that the surface is hydrophilic i.e. wettable with a contact angle of 30° or less. This is possible by the addition of various hydrophilising agents e.g. 1% glycerine stearate or Crodamol., Fleroxacin, Lubrophos. The hydrophilising agents have to be adapted to the polymer. Antimicrobial cables have been produced by incorporation of 2 % Molybdenum Trioxyd in thermoplastic polyurethane which is already sufficient hydrophilic on the surface. The investigation of antimicrobial activity has been performed by the rollon culture technique. 5cm pieces of cable containing 2 % of molybdenum trioxide have been immersed into a suspension of S. aureus ATCC 25923 in a concentration of 109CFU/ ml for 6 hours. Thereafter the pieces have been rolled over an agar plate (Oxoid Agar containing 5 % sheepblood) Then sample has been added to a sterile empty Saarsted vial. This procedure has been repeated in 3 hourly intervals until 12 hours. It has been shown that the initially contaminated samples did not contain any microorganisms after 3 hours and thereafter. In an additional experiment samples of cables produced in 2002 have been investigated with the same method. These 18 years old samples showed identical results compared to the original samples.
Public transportation especially air transport transformed the world into a “global village” where newly emerging pathogens but also multiresistant microorganisms travel several 1000 miles within days. It became evident that conventional measures e.g. disinfectants don’t meet the necessary requirements. We have to take care of these new challenges with immediate innovative and ambitious measures. Permanent genuine germfree surfaces can contribute to solve the problem. Catalysts e.g. transition metal oxides function as eager electron donors in polymers and coatings and transform ambient water into acid water molecules, free radicals e.g. oxygen radicals but also hydroxyl radicals as in situ generated biocides. The most effective antimicrobial property comes from the positive Zeta potential, a positive electric charge in μm distance attached on the surface. Electro negative charged microorganisms are attracted and a disruption of the phospholipid bilayer of microorganisms occurs within minutes leading to an eradication of a broad spectrum of microorganisms. This technology also works against microorganisms embedded in a biofilm. The additive can be applied into various polymers, paints and coatings - the activity of these in situ generated biocides is lasting and has been documented for minimum 10 years. 1000 cleaning cycles with water and a detergent don’t impair the antimicrobial activity.


