Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Regina Przywara1, Marcin Chutkowski2, Mateusz Przywara2 and Wojciech Zapała2*
Received: July 15, 2022; Published: August 02, 2022
*Corresponding author: Wojciech Zapała, Department of Chemical and Process Engineering, Chemical Faculty, Rzeszów University of Technology, Powstańców Warszawy 6, 35-959 RZESZOW, Poland
DOI: 10.26717/BJSTR.2022.45.007209
In this work the preliminary experiments on the quantitative analysis of sodium salt heparin obtained from animal extracts in different chromatographic systems have been presented. One HPLC column filled with charged amine phase and two UHPLC columns filled with non-polar C18 phase and porous graphitic carbon phase have been tested. The introductory results concerning the effects of eluent composition and eluent pH have been briefly discussed. The results indicate the satisfactory sensitivity/selectivity of the proposed separation systems. In addition, the obtained analysis times are much shorter than those of the pharmacopoeia method, which will probably allow for quick analyzes under industrial conditions.
Keywords: Adsorbent; Heparin; Liquid Chromatography; Quantitative Analysis
Heparin (Figure 1) is a highly sulfated, linear polysaccharide consisting of a repeating disaccharide unit that contains iduronic acid, glucuronic acid and glucosamine residues. Heparin is well known for its potent anticoagulant activity, but it also has an additional broad range of biological properties due to its high negative charge and heterogeneous molecular structure [1]. Pharmaceutical grade heparin is extracted from animal tissues primarily from porcine mucosa. Most currently used heparins have been purified from the mucosa of porcine and bovine intestines and, to a lesser extent, from bovine lungs that are harvested at a slaughterhouse. Modern medical procedures, including thrombotic treatments such as deep vein thrombosis and extracorporeal therapies such as kidney dialysis and blood oxygenation, the use of catheters and intravascular fistulas have increased the demand for heparin production [2]. One method for the determination of heparin is the spectrofluorimetric method, which is used to determine trace amounts of heparin in biological samples [3]. The HPLC coupled with resonance light scattering detection was developed for separation and determination of heparin in plasma [4]. The HPLC analysis of heparin can be carried out using a polymer-based anion exchange column [5]. Ion chromatography separation techniques can be powerful tools in the separation of highly charged polydispersed analytes such as heparin and related compounds [6]. For the determination of heparin in drugs, gradient liquid chromatography on anion-exchange resin is recommended by the European Pharmacopoeia [7]. However, the disadvantage of this solution is a relatively long analysis time - about 26 min. The aim of this study was to check the applicability of various stationary phases (amine, C18, graphitic carbon) for the quantitative evaluation of sodium salt of heparin content in raw animal extracts.
Column and Instrumentation
The investigations were conducted using one HPLC column (Merck LiChroCART Purospher STAR NH2, 125 mm x 4.0 mm, 5μm particle size, 120Å pore size and 330 m2/g surface area) and two UHPLC columns: Thermo Scientific Hypercarb Porous Graphitic Carbon HPLC (100 mm x 2.1 mm, particle size 3μm, pore size 250 Å and surface area 120m²/g), NUCLEOSIL 120-3 C18 (100 mm x 4.0 mm, 3 μm particle size, 120 Å pore size, and surface area 200m²/g). The UHPLC system consisted of UltiMate 3000 RS Pump, Ultimate 3000 RS Autosampler, Ultimate 3000 RS Column Compartment, UltiMate 3000 RS Diode Array Detector and Merck solvent degasser (model L-7612). This chromatograph was used in systems with the C18 and carbon columns. The HPLC system consisted of Primaide Merck–Hitachi pump (model 1110), Primaide Merck–Hitachi UV detector (model 1410), Primaide Merck–Hitachi column oven (model 1310).
Chemicals
Standard 1 of heparin sodium salt was obtained from Toronto Research Chemicals inc. (2 Brisbane Road, Toronto, ON, Canada M3J 2J8, TRC Canada), and Standard 2 of heparin sodium salt was obtained from European Pharmacopoeia (EP) References Standard (heparin for physico – chemical analysis, Council of Europe – EDQM CS 30026 F-67081 Strasburg, Merck Life Science). For the experiments, two separately manufactured heparin extracts (derived from porcine intestinal mucus) in the form of sodium salts were also used. These extracts were obtained from Animex Foods Poland. All chromatographic grade organic solvents were purchased from Merck. As mobile phases the ACN – water systems containing different volume fractions of the ACN have been used (note, that only selected results are presented in this study). As eluent was also used 90% ACN – acetate buffer with pH 5. All buffer ingredients, i.e. sodium acetate and acetic acid were purchased from Chempur. An ultrasonic bath was used to degas the mobile phases for 5 minutes immediately after mixing. Distilled, deionized, and demineralized water was prepared on SolPure-78Z (ELKAR) water deionizer.
Methodology
All measurements were carried out under isocratic conditions at an eluent flow rate of 1 ml/min for a given eluent composition and column temperature equal 20°C. The injection volume (samples dissolved in the mobile phase) was 0.02cm3 of working standard solutions (20μg/cm3). At the beginning of each set of experiments, the column was equilibrated at stable temperature by washing with approximately 20 column hold-up volumes of the fresh mobile phase. Peaks were recorded at 202.0 nm (recommended by the European Pharmacopoeia [7]). The experiments were repeated twice for each mobile phase composition and the measurements were reproducible. Due to the initial nature of the investigations, in this study the interpretation of the results was limited only to the visual assessment of the obtained chromatograms.
The exemplary results for the tested systems with eluents containing 90% ACN are shown in Figure 2. In Figs. 2A and 2B, the chromatograms obtained with the use of the UHPLC chromatograph are presented. The application of the non-polar C18 column (Figure 2A) allows to obtain a very high selectivity/sensitivity of the separation and a significant (compared to the method presented in the European Pharmacopoeia) reduction of the analysis time to about 8 minutes. An even greater reduction in the analysis time (to about 3 min) can be achieved by using a carbon column (Figure 2B). However, the selectivity/sensitivity is in this case slightly poorer – see Figure 2B. Compared to the carbon column, slightly better results were obtained in the system with the amine column and non-buffered mobile phase (HPLC system) – see Figure 2C. However, the analysis time is longer (about 15 min) in this case. The use of a buffered mobile phase with pH 5 shortens the analysis time to about 12 min and slightly reduces the separation selectivity (Figure 2D).
Figure 2.The example chromatograms of two standards and two extracts of sodium salt heparin in different chromatographic systems (description in the text).
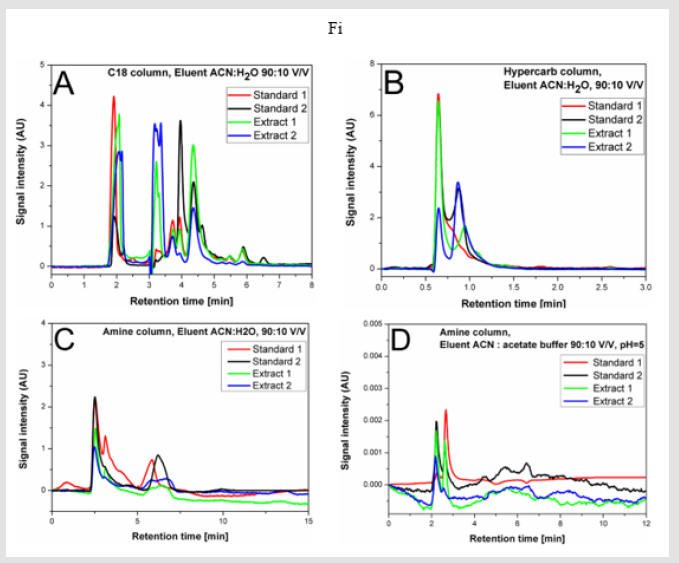
The other experiments conducted in the tested columns (data not presented in this communication) indicate, that the use of eluents with different ACN contents does not significantly affect the selectivity/sensitivity of the separation. Generally, with lower ACN contents in the eluent, only the analysis times are extended. Based on the presented here preliminary results, it can be concluded that the quantitative analysis of the heparin content in animal extracts can be performed in the tested columns/systems under isocratic conditions using both HPLC and UHPLC chromatography, while maintaining the similar quality of separation as using other (literature known – e.g. [2-7]) chromatographic techniques. The best results are obtained with an eluent containing 90% ACN and 10% water. Note that, the presented preliminary results allow to obtain a shorter analysis time than stated in the European Pharmacopoeia.
The preliminary results presented in this communication indicate both the satisfactory selectivity and sensitivity of separation obtained using isocratic conditions in columns filled with both non-polar (C18), porous graphitic carbon phase and also polar (amine) stationary phases. It is important that the use of UHPLC chromatography significantly shortens the analysis times. Besides there is no need to use gradient techniques, which makes also the analysis easier and faster. The results presented here indicate also that the analysis time can be significantly shortened compared with these described in available literature and the European Pharmacopoeia. The obtained results can probably be used for fast quantitative analysis in companies producing crude heparin from animal raw materials. Obviously, the presented results should be treated as preliminary and require further studies.
The authors have declared no conflict of interest.
The authors confirm that the data supporting the findings of this study are available from the corresponding author upon reasonable request.


