ABSTRACT
The endocannabinoid system is made up of cannabinoid receptors and their ligands, enzymes, and subsequent metabolisms. Cannabinoids are either synthetic or natural compounds found in the Cannabis sativa family, that exert their effects via binding to the cannabinoid receptor. Most recent studies indicate the need to search for compounds that selectively target cannabinoid receptors that are located outside of the blood-brain barrier, thus allowing pain relief. We look at several clinical trials to evaluate the efficacy of medical cannabis as the management of pain in cancer patients through its interaction with the endocannabinoid system. Data was extracted using online research engines to investigate the main question, with six studies obtained based on using cannabinoids as a form of pain management to treat cancer-related pain. In five out of the six clinical trials, most patients experienced a reduction in pain as per the reported results for each, with some trials reporting P values of 0.274 and 0.0854. Some trials have analysed how cannabis during earlier treatment stages of cancer symptoms can play a role in pain management and should be investigated in further trials.
Abbreviations: PAG: Periaqueductal Gray Matter; CNS: Central Nervous System; THC: Tetrahydrocannabinol; MAPK: Mitogen-Activated Protein Kinase; PRIOR: Preferred Reporting Items for Overviews of Reviews; RCT: Randomised Controlled Trials; NRS: Numerical Rating Scale; VAS: Visual Analog Scale; MC: Medicinal Cannabis; CBD: Cannabidiol; P: P value
Introduction
The endocannabinoid system is a neuromodulatory system that plays an important role in pain management and has recently been the focus of research publications [1]. The endocannabinoid system is made up of cannabinoid receptors and their ligands, enzymes, and subsequent metabolisms. There are four receptors that have been discovered throughout the body: CB1, CB2, WIN, and abnormal cannabinoid receptors [2]. These receptors are G protein receptors with seven folded transmembrane helices that function in signal transduction [2,3]. CB1 receptors contribute to the modulation of neurotransmitter activities in the central nervous system (CNS) with high concentrations seen in the basal ganglia, cortex, hippocampus, cerebellum, thalamus, amygdala, midbrain periaqueductal gray matter (PAG), as well as the substantial gelatinosa of the spinal cord on myelinated A-fibers and a small number of C fibers. Furthermore, CB1 receptors can also be expressed to some extent on cells of the immune system, namely mast cells and macrophages [2,4,5].
CB1 receptors may initiate and influence memory, mood, sleep, appetite, motor control, and pain sensation. They also influence the release of several neurotransmitters including dopamine, noradrenaline, serotonin (5-HT), gamma-aminobutyric acid,and glutamate [6,5]. Most CB1 receptors are located on pre-terminal axon segments and inhibit synaptic transmission through the regulation of neurotransmitter release by hindering voltagesensitive calcium channels and the activation of potassium channels [2,7]. On the other hand, CB2 receptors are commonly located on immune cells like reactive microglial cells and hepatic Kupffer cells, in addition to osteoclasts, osteocytes, keratinocytes and splenic cells. Moreover, these receptors are found in some areas of the CNS such as cerebellar granule cells [2,4,5]. CB2 receptors are involved in preventing cytokine production and hence the attenuation of pain and inflammation [2,5,8]. An array of treatments exist for cancer pain including pharmacological interventions such as NSAIDs and opioids, but a new potential alternative treatment is the use of cannabinoids [3,5].
The potential prospective shift from opioids to cannabis may be owing to the fact that chronic opioid use can lead to unwanted side effects such as severe constipation, mental clouding, gastrointestinal symptoms, endocrinopathies, fatigue, infertility, reduced libido, osteoporosis, menstrual changes, neurotoxicity, sleep disorders, and hyperalgesia. Additionally, abuse of opioids diminishes the quality of life both in cancer as well as non-cancer patients [9]. Similarities between opioids and cannabinoids can be seen as they initiate the same pharmacologic effects such as antinociception, hypothermia, inhibition of locomotor activity, hypotension, and sedation [10]. Cannabinoids are either synthetic or natural compounds found in the Cannabis sativa family, that exert their effects via binding to the above-mentioned receptors [6,8]. Endocannabinoids are bioactive lipids and arachidonic acid derivatives made within the body that act on the cannabinoid receptors [6,7,11]. It has been reported that cannabinoid agonists can even enhance the potency of opioids, reportedly due to their parallel signal pathways [10].
The CB1/CB2 agonists are used for the improvement of chemotherapy-induced nausea, vomiting, and management of neuropathic pain in multiple sclerosis and advanced-stage cancer patients [11,6]. Many CB1/CB2 agonists, namely Δ9 -tetrahydrocannabinol (THC), N-arachidonoylethanolamine (anandamide, AEA), and 2-arachidonoylglycerol (2-AG) inhibit the presynaptic release of neurotransmitters from glutaminergic and GABAnergic neurons, thus aiding in pain relief [3,2,5]. Other endocannabinoid and endocannabinoidlike ligands worthy of mention are O-arachidonoylethanolamine (virodhamine), 2-arachidonoylglycerylether (noladin ether), N-arachidonoyldopamine (NADA), N-docosahexaenoylethanolamine (DHEA), N-eicosapentaenoylethanolamine (EPEA), whereby all are acquired from unsaturated fatty acids that are grouped based on their slight structural differences in hydrocarbon chains [11].
The downside of CB1/CB2 agonists is their psychoactive effects due to their ability to cross the blood-brain barrier and act on many regions of the brain and brain stem [12,2]. The ramifications of the psychoactivity of CB1/CB2 agonists, although rare, include euphoria, disorders of sleep, hyperactivity, irritability, agitation, and may be accompanied by auditory-verbal hallucinations and short-term memory loss while it may aggravate a pre-existing mental disorder [13]. Most recent studies indicate the need to search for compounds that selectively target CB2 receptors along with CB1 receptors that are located outside of the blood-brain barrier, thus allowing pain relief without the abovementioned psychoactive adverse effects. By specifically working through the mitogen-activated protein kinase (MAPK) pathway, CB2 agonists reduce several types of pain, including neuropathic pain and cancer pain. They also have anti-inflammatory, muscle relaxant, and neuroprotective activity [5,12,2]. In this systematic review, we look at several clinical trials to evaluate the efficacy of medical cannabis as the management of pain in cancer patients through its interaction with the endocannabinoid system and the subsequent neuromodulatory effects.
Methods
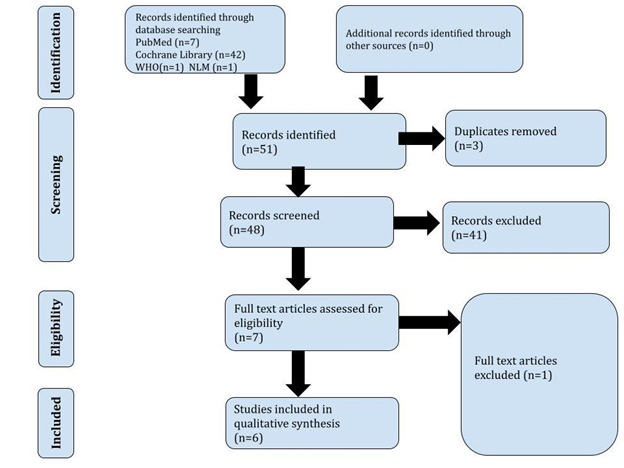
This document record is reported under the PRISMA-P checklist, 2015 [14]. We worked as per the directions of the Preferred Reporting Items for Overviews of Reviews (PRIOR) guidelines for overviews of reviews of healthcare interventions [15]. We used the latest PRISMA guidelines for reporting the review in case of any updates to the used protocols, as shown in Figure 1.
Inclusion Criteria
Oncology-related pain and pain management using cannabinoids in natural and synthetic forms was the main focus of each paper reviewed. Our reviewers considered various cancer types and stages. Cannabinoids affect cannabinoids administered via various routes; that is, oral mucosal spray, oil formulation, pills, liquids, inhaled formulation, and topical formulation were also reviewed by the team. Clinical trials published within 2016– 2022 relevant to the stated review, with text reported in English, were considered for data extraction and review. The clinical trials included Randomised Controlled Trials (RCTs), Double-Blind Trials (DBTs), and observational studies. The clinical trials reviewed had adult participants (men and women) aged 18–65 years. The Cochrane review [16], Medline (PubMed) [17], the National Library of Medicine, and the International Clinical Trial Registry Portal facilitated by the World Health Organization [18] were the search engines utilized. The keywords used on search engines were “endocannabinoid system”, “pain-management”, “oncology”, “cannabis” and “cancer”. Out of 49 clinical trial reports obtained, we could only use nine, which offered full open access to the full text. Our reviewers have reported all clinical outcomes and adverse effects.
Exclusion Criteria
Our reviewers have excluded studies that involved participants aged under 18 or over 65 years of age and outcomes of trials that are self-reported rather than statistically or objectively measured. We eliminated non-clinical trials and reviews of studies and editorials based on previous papers before 2016. Therapeutic use of Cannabinoids in Non-cancer Related Pain and papers in which trials have been terminated or published papers that were withdrawn were excluded. Reviewers did not consider patients who had been using cannabis or cannabis-derived products before the clinical trials.
Information Sources and Search Strategies
Our search process was performed according to the principles of the Cochrane Handbook for Systematic Reviews of Interventions and recommendations for conducting Overviews of Systematic Reviews [19]. The relevant papers were identified using the keywords mentioned under the inclusion criteria on electronic databases, namely PubMed, Cochrane Review, WHO, and the National Library of Medicine databases. The searches were limited to the unterminated clinical trials published in English between 2016 and 2022. References have also been located for relevant articles taken into consideration. To ensure that emerging evidence has been covered, we also searched for recently published or ongoing/planned RCTs in the Cochrane CENTRAL database and significant clinical trial registries relevant to our review process (the exact periods, for apparent reasons, will depend on the timing when the searches were performed in published systematic reviews) and have also set up citation alerts in MEDLINE and EMBASE (Via the CENTRAL search strategy and use of additional search filters for RCTs).
Selection Process and Data Collection Process
Review Selection: Two reviewers [OD and VV] have independently screened all the titles and abstracts. A total of fifty one clinical trial reports were collected; forty-two from Cochrane, seven from Medline, one from WHO, and one from the National Library of Medicine. The disagreements have been resolved by discussion.
Data Extraction: The data has been extracted by three independent reviewers [OD, VV, and MY] from each chosen anchoring systematic review, which includes:
• Review characteristics: year published, number of included RCTs, a summary of intervention, and comparator.
• Type of patient (oncology patient), age group, and gender • All clinical outcomes and adverse events were reported.
• Certainty of evidence.
• Assessments are at risk of bias.
To present the findings of this systematic review, we extrapolated data that correlates with the main title. We collated and grouped the data using the following specific variables: the types of clinical trials conducted; the gender of the participants; the dosage of intervention; the route in which the intervention was administered; the response of the participants; and the number of participants included within all papers. Additionally, we ensured that all variables were within the range of our inclusion criteria to achieve the maximum outcome for our systematic review.
Primary Outcomes
The primary outcome of our research paper was to determine whether there was a reduction in pain amongst participants receiving various types of cannabinoids, reviewed by several pain grading criteria. For example, the pain numerical rating scale (NRS) and the visual analog scale (VAS) were two types of grading criteria used to conclude our research and formulate a definite conclusion adequately.
Secondary Outcomes
The secondary outcome of our systematic review were variables that were determined alongside the primary outcome:
• The total number of participants per study
• route of administration [including oral, inhaled, topical, capsule, oil, liquid].
• Dosage of the drug [in mg/dL or at specific times throughout the study].
• Mortality of patients [throughout the duration of each study]
• Withdrawal from the study [due to side effects or unintentional circumstances].
• Side effects [including nausea, vomiting, dizziness, constipation, somnolence, anemia, anxiety, insomnia, euphoria]
Preparing for Synthesis
Reviewers grouped each clinical trial accordingly to prepare the data collected. For example, all RCT trials were analysed simultaneously so that specific patterns and correlations could be depicted amongst both. Using other systematic reviews as a basis to complete ours, we decided to utilise a table to display all the data within the papers sourced from the internet. We were able to group the variables from each study in a concise manner, comparing the differences and stating the similarities between each of the clinical studies as part of the meta-analysis.
Tabulation and Graphical Methods
The initial search yielded promising results, displayed within a summary table created using Microsoft Excel 2020. Our reviewers uploaded data from all six clinical trials into the table, and the data within was extrapolated and grouped to formulate the results for this systematic review. Variation amongst each sub-group variable from all six clinical trials was apparent. Thus, graphical documentation was difficult to generate at this given time.
Methods to Explore Heterogeneity
A tabulation was created to determine the heterogeneity amongst the data. As a result of formulating our meta-analysis, there was limited heterogeneity amongst the different clinical trials conducted on the use of cannabinoids to treat cancer-related pain. Five out of six studies yielded similar results, which only highlighted the strength of our initial research strategy.
Assessment of Bias Risk
To reduce the risk of bias in our systematic review, we utilised the Risk of Bias in Systematic Reviews (ROBIS) tool [20]. The risk of bias has been minimized further as all the reviews were clinical trial papers.
Reporting Bias
To eliminate and reduce the emergence of bias within our systematic review, we were sure to include only studies that followed the relevant criteria of meeting clinical trials and presented evidence of statistical analysis. Any studies which did not meet this criterion were highlighted and further explored.
Results
Type of Research
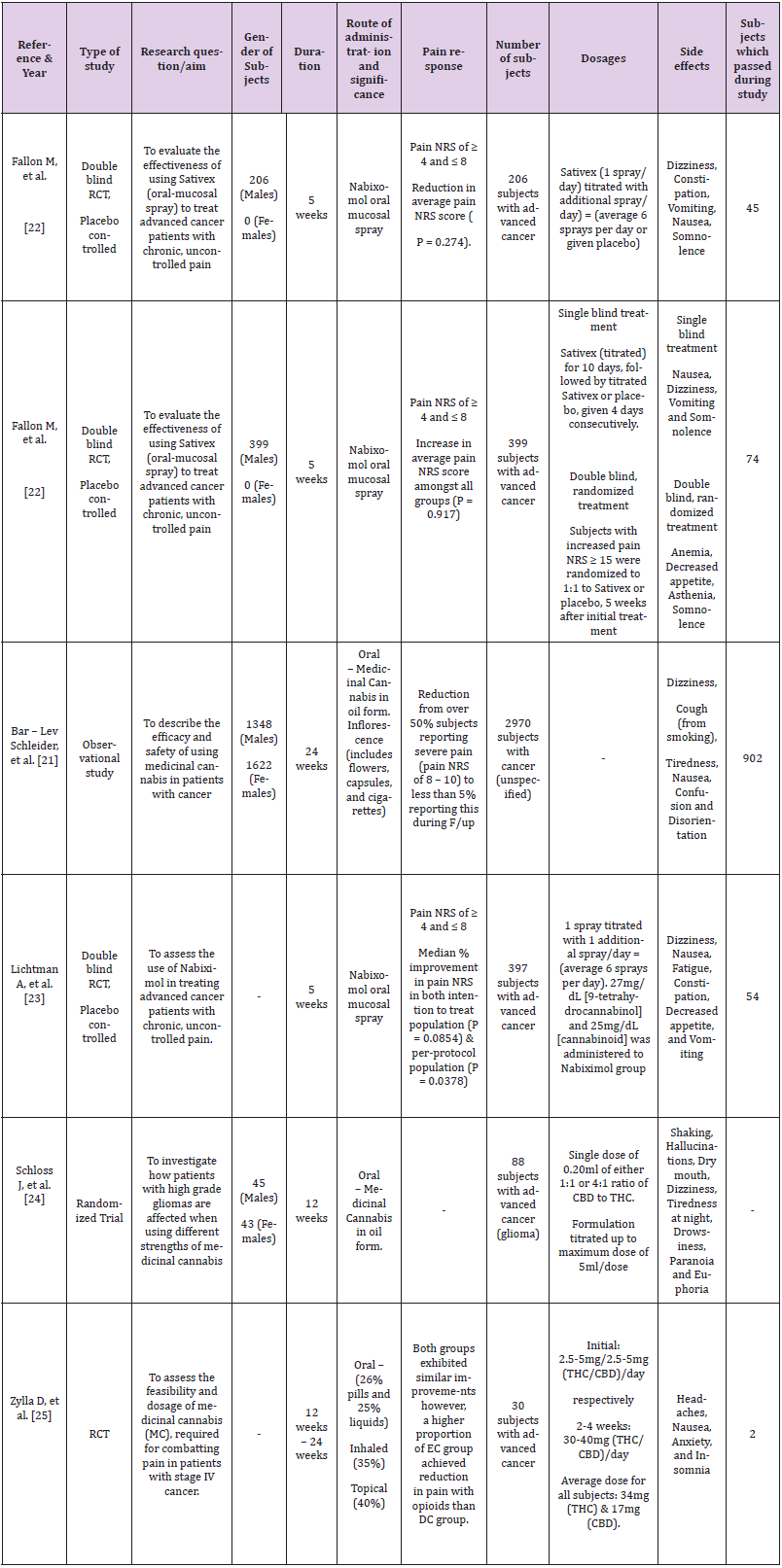
Using online research engines to investigate the main question of this systematic review, six studies were obtained based on using cannabinoids as a form of pain management to treat cancer-related pain. Out of the six populated studies, three consisted of trials that were double-blinded, randomized, and placebo-controlled (Fallon M et al., and Litchman et al.,). Two obtained papers consisted of randomised controlled trials (Schloss J et al. and Zylla D et al.) conducted in 2021. The remaining report by Bar-Lev Schleider et al. was an observational study conducted in 2018. Table 1 below summarises the papers and associated clinical trials used to determine whether the administration of cannabinoids to treat cancer-related pain is an effective treatment.
Type of Research
Using online research engines to investigate the main question of this systematic review, six studies were obtained based on using cannabinoids as a form of pain management to treat cancer-related pain. Out of the six populated studies, three consisted of trials that were double-blinded, randomized, and placebo-controlled (Fallon M et al., and Litchman et al.,). Two obtained papers consisted of randomised controlled trials (Schloss J et al. and Zylla D et al.) conducted in 2021. The remaining report by Bar-Lev Schleider et al. was an observational study conducted in 2018. Table 1 below summarises the papers and associated clinical trials used to determine whether the administration of cannabinoids to treat cancer-related pain is an effective treatment.
Table 1: Results of various clinical trials on the impact of using cannabinoids, as a form of treatment to manage oncology related pain.
Duration of Treatment
Various time frames were used during the clinical trial in each study being reviewed. Bar-Lev Schleider et al. [21] report a sixmonth duration of treatment with cannabinoids due to the study type, which required a more extended period of observation. The study states a one-month follow-up and a six-month review to determine the efficacy of treatment. This contrasts with the nature of the other studies, as those clinical trials required intervention. Trials conducted by Fallon M, et al. [22] study as well as Lichtman A, et al. [23] had a similar period of 5 weeks as well as almost identical results in terms of improvement in pain as reported by patients using the Numerical rating scale (NRS) for pain. Schloss J, et al. [24] and Zylla D, et al. [25] also share a similar time frame of 12 weeks and three months, respectively. However, their results are not comparable as Schloss J et al. focuses on pain reduction in the context of a general reduction of cancer symptoms. This is further differentiated by the fact that Zylla D et al. do not state the use of a specific pain scale but rather the patients’ reporting of pain improvement and achievement of targets as determined by patients.
Patient Population
The variety seen in the studies also extends to the number of patients that are included in the specific studies mentioned. Bar- Lev Schleider, et al. [21] included many more patients than the other studies, as the trial method utilised was more observational than interventional. The study recorded 3845 patients receiving a cannabis licence as indicated for usage in cancer indications. Patients were noted to have a variety of cancers and differences in staging. 2.1% of patients were reported dead before treatment initiation. 3.7% of patients who received the license, however, opted out of treatment. 0.2% changed to a different cannabis supplier, and 94.1% of patients initiated treatment protocols. Studies by Lichtman A, et al. [23] and both trials by Fallon M, et al. [22] focused on patients diagnosed with advanced cancer while demonstrating a similar sample size, which once again results in similar findings in terms of the reported pain response from the patients. Lichtman, A et al. [23]included 542 patients that were screened, but only 397 met the inclusion criteria.
Of these individuals, 199 were placed on nabiximols while the remaining 198 were added to the placebo group. 20.1% and 17.7% withdrew from the study due to adverse events occurring during treatment in the nabiximols and placebo groups, respectively, while 13.6% of patients died during the study. Fallon M, et al. [22] conducted two studies in total, and in the first one, 528 patients were screened for enrollment, with only 399 patients deemed eligible for the study. Of these, 200 patients were randomised to Sativex and the rest to the placebo group. 3.2% and 20.6% of patients from both the Sativex and placebo groups withdrew from the study. The most common causes were a negative outcome for discontinuation (19.0% in comparison to 14.6% in the Sativex and placebo groups, respectively) and consent revocation (19.5% vs. 4.0%). In the Sativex study, 10% of patients died, while the placebo group recorded 12.6% of deaths. In total, 68.0% of Sativex patients and 79.4% of placebo patients completed the study.
Dosage and Route of Administration
Patients were given an initial dose of one Nabiximols/Sativex oromucosal spray in both studies by Fallon, M et al. [21] and Lichtman, et al. [22], which was gradually increased or titrated according to the patient’s tolerance capacity. In the study by Zylla D, et al. [23], patients were given cannabis as pills, inhalation products, sprays, and oral solutions, starting with 2.5 to 5 mg THC/ CBD and gradually increasing to a daily maintenance dose. Bar-Lev Schleider, et al. [24] used two methods of administration: oil and inflorescence, which included flowers, capsules, and cigarettes, which were a new form of cannabis not used in any of the previous studies [21-25]. Schloss J, et al. [25] gave patients a single dose of oral cannabis oil, which was similar to one of the routes taken by Bar-Lev Schleider , et al. [24], but with different dosages and ratios based on a 1:1 and 4:4 ratio of THC; CBD starting at 0.20mL and going up to 5mL in one dose, as observed in Table 2.
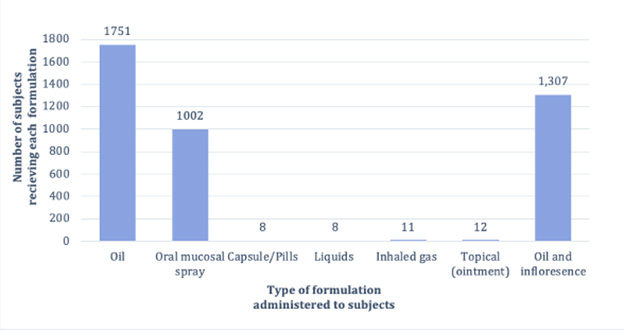
Figure 2: Percentage of patients given medicinal cannabis, through specific routes of administration (%).
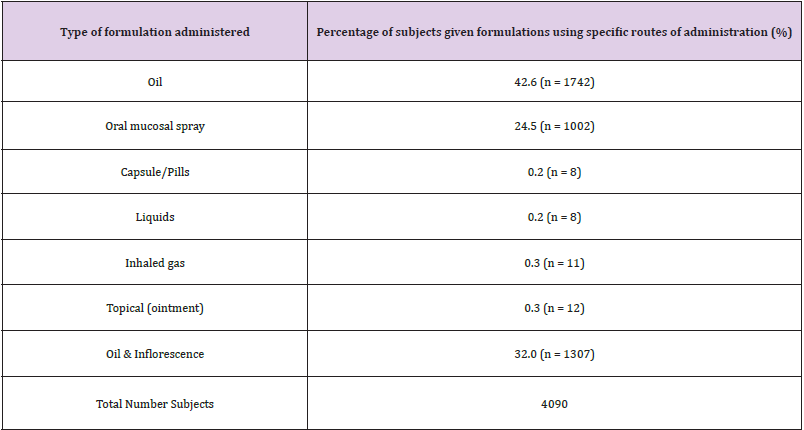
Table 2: Types of administration routes utilized across all studies, to administer formulations of medicinal cannabis.
Withdrawal of Patients During the Study
In the Fallon, et al. [21] study, 200 patients were randomly assigned to Sativex and 199 to placebo. A total of 64 (32.0%) patients withdrew, with 38 (19.0%) experiencing adverse effects. Twenty people (10%) died during treatment, post-treatment, or followup. There were 41 (2.6%) withdrawals from the placebo group, 29 (14.6%) due to adverse effects, and 25 (12.6%) patients died. In study 2, 406 patients enrolled; 198 (48.8%) withdrew; 71 (17.5%) experienced adverse effects; 42 died during the first titration phase; and the remaining 206 were randomly assigned to Sativex or placebo. There were 103 people in each of the Sativex and placebo groups. Due to adverse events, 25 (24.3%) of Sativex patients and 15 (14.6%) of placebo patients withdrew, and 32 (15.5%) died during the second randomised phase, with 23 (22.3%) from the Sativex group and 9 (8.7%) from the placebo group. Lichtman, et al. [22] enrolled 542 patients and randomly assigned 397 of them to Nabixomol (199) or placebo (198).
Patients from the Nabixomol group (58.1%) and the placebo group (48.2%) dropped out of the study. Due to adverse events, 40 (20.1%) and 35 (17.7%) patients from the nabiximol and placebo groups withdrew from the study. 27 (13.6 percent) died in each group. Zylla, et al. [23] assigned 30 people to one of two groups: early cannabis (EC) or delayed cannabis (DC). The number of patients who dropped out due to adverse effects is unknown. One patient died during treatment in the EC group. After a 3-month follow-up, five members of the EC group were removed due to noncompliance with the study, and one died. Due to non-compliance with the study, two members of the DC group were removed, and one died. The Schloss, et al. [25] study evaluated 921 patients, with 88 eventually enrolled. 27 patients dropped out, and 92 died before or during the study. After 12 weeks, 3 patients in group A died, and 5 in group B died. The number of patients who withdrew due to adverse effects is unknown. Bar-Lev Schleider, et al. [24] studied 3845 cannabis-licensed patients and found that 79 (2.1 percent) died before treatment.Six months into the trial, 658 (22.1 percent) of the 2968 patients died; 290 (9.8%) withdrew from treatment; 19.3% experienced side effects; and 1997 (67.3%) patients continued treatment.
Pain Response
While acquiring papers concerning the main title of our systematic review, the severity of pain experienced by patients across all papers was graded using several different analytical techniques. Trials conducted by Fallon M et al. and Lichtman A, et al. [22,24] both utilised the universal pain numerical rating scale (NRS) to assess the quantity of pain felt by patients (on a scale from 0/no pain to 10/chronic pain) after taking specific formulations of medicinal cannabis. In both studies by Fallon M et al. (“Study 1”) and that of Lichtman et al., there was an overall reduction in the average pain (NRS) score, with most patients reporting a pain (NRS) score in the range of 4 and 8. In contrast, “Study 2,” also carried out by Fallon M et al., showed an overall increase in the average pain (NRS) score amongst all patients [21]. Moreover, the study conducted by Bar-Lev Schlieder et al.Utilised a similar pain rating scale, referred to as the visual analogue scale (VAS), to assess the quantity of pain amongst patients. As a result, there was a reduction in pain reported in more than 50% of the patients in the Bar-lev Schilder et al. clinical trial [23]after taking medicinal cannabis.
Additionally, one of the newer trials reported in this review, conducted by Zyllan D et al, utilised the VAS pain rating scale to document participant pain within the clinical trial. However, the clinical study by Zyllan D et al. reported a pain reduction scale that averaged between 3 and 6 [3]. In addition, patients within the Zyllan D et al. clinical trial were also asked to complete a survey to report specific treatment and induced side effects experienced during the study and to keep a form of documentation on the quantity of cannabis-based products ingested. The remaining study by Schloss J, et al. [25] assessed the efficacy of medicinal cannabis in treating pain and other oncological-related symptoms. Unlike all the other clinical trials mentioned within this subtext, researchers did not put pain at the forefront of the study conducted by Schloss et al. However, there was a reported reduction in pain experienced by the patients of the study (P = 0.019).
Test administered with Side Effects
Across all the studies within this review, the majority of patients reported several similarly stated side effects. In “Study 1” by Fallon M et al., the treatment-related side effects reported amongst both control and placebo groups included nausea (18%), dizziness (21%), and somnolence (24%). Similarly, in part A of “Study 2” by Fallon M et al., patients involved in the single-blinded trial also reported similar adverse effects. In contrast, in part B of “Study 2” by Fallon M et al., where patients (control and placebo groups) took part in a double-blinded randomised trial, the only treatmentinduced side effect reported amongst the patients was somnolence (6%). In addition to the findings within “Study 2” by Fallon M et al., more severe adverse effects were reported by the patients, such as weight reduction (11%), anaemia (13%), asthenia (12%), and decreased appetite (9%) [22].
The study by Litchman A et al. consisted of reported side effects that replicated those found in both studies by Fallon M et al. The treatment-induced side effects identified by Schloss J et al. and Zylla D et al. were more psychological [24,25].
For instance, adverse effects such as anxiety and insomnia were reported by patients receiving oral (26% pills and 25% liquids), inhaled (35%), and topical (40%) forms of medicinal cannabis to combat pain during the Zylla D et al. clinical study [3]. Paranoia and drowsiness were among the side effects reported by patients with gliomas taking titrated doses of THC/CBD (maximum 5ml) [25]. Similarly, in the study by Bar-Lev Schleider et al., patients reported experiencing confusion and disorientation. However, an additional side effect, being that of coughing, has been reported by some patients taking their cannabinoid treatment via using a cigarette [24]. It is important to note that throughout the studies, side effects were more prevalent amongst the patients who were given cannabinoids than those receiving a placebo.
Discussion
Patient Population
The population included in this systematic review was a large and heterogeneous group. The two trials under Fallon M, et al. [22] included only males, while two of the other six studies included a mixture of males and females. Bar-Lev Schleider [21] also contained a much larger number of patients and included a variety of patients, not necessarily limited to advanced cancer. This may explain why that study reported a more general indication of what the patients reported as a reduction in pain than the other studies. However, it should be noted that the report on pain reduction can also be attributed to the fact that patients varied in cancer types and staging. Lichtman A, et al. [23] and Fallon M, et al. [22]both reported a similar number of patients and thus reported an almost identical pain response, with both studies reporting pain reduction on the Numerical Rating Scale (NRS) below or equal to 8, but still above 4. Some studies, however, such as Schloss J, et al. [24], opted for a much smaller sample size, and pain management was a secondary priority, with an overall improvement of the cancer symptoms being the primary. Zylla D, et al. [25] had the smallest sample size and reported a different pattern of pain reduction. This is evident as there was no reduction in the mean pain score; however, patients reported meeting their personalised pain goals.
Duration of Treatment
The studies included within this systematic review varied within the timeframe that they followed, which has impacted the results collected by the study. This creates a challenge as the studies under consideration all used cancer patients as their patients, with five out of the six studies in question specifically using patients with advanced cancer. Thus, due to the nature of the cancer progression, some of the patients in the study passed away. Bar-Lev Schleider, et al. [21] had a significantly more extended period than the other studies listed and recorded a higher death percentage. This can be explained by the fact that the study is observational, requiring a more extended timeframe to establish notable differences in results because no variables are controlled and no intervention is applied. This study also reflects that most of the patients who did not drop out or die throughout the six months reported an improvement in their symptoms, with less than 5% reporting severe pain (8 on the visual analogue scale).
However, this becomes less comparable with the other studies when we consider that the subjects enrolled were not advanced cancer subjects, which is what is included in the other studies investigated. The study may also show a variation in comparison with the other studies in the final results as it had a more significant number of subjects that did not continue, whether due to death (902 subjects) or discontinuing treatment (290 subjects at six months). In contrast, Fallon M, et al. [22]and Lichtman A, et al. [23] had an identical timeframe and, as such, had almost identical results. While Zylla D, et al. [25] and Schloss J, et al. [24] shared an almost identical timeframe of 12 weeks, they did not share similar results in the pain response section. This can be attributed to the fact that the former did not use a scale to monitor the reduction in pain. At the same time, the latter opted to focus on the reduction in all of the cancer symptoms rather than the reduction in pain response alone.
Mode of Administration and Dosage
Cannabis can be administered in a variety of ways to alleviate pain. Its efficacy and side effects are all affected by the route, form, and dosage. In this study, we compared oral mucosal spray, vaporised oil, oral solution, pills, and inflorescence (flowers, capsules, and cigarettes). Dosing is a challenge in all studies because there are no established or standardised dosages. It varies depending on the individual, the type of cannabis used, as well as the method of administration. One of the study’s primary goals was to determine a standard dose and the best route and form of administration for a patient to achieve pain relief. This was made more difficult because Bar-Lev Schleider does not specify the dosages and daily limit of cannabis consumed by their patients. Bar-Lev Schleider’s review includes studies that demonstrate the efficacy of cannabis via two routes of administration: oil and inflorescence.
This study [24]discusses the cannabis strains and their THC and CBD ratios. In terms of duration, formula, and dosages of cannabis used, Fallon [21]and Lichtman [22] had similar study structures. In contrast to Fallon’s and Lichtman’s studies, the patients in Zylla D, et al. [23] received cannabis in the form of vaporised oil, oral solution, and pills. Pills and liquids were the most commonly used oral products (51%), inhalation (35%), and topical (14%), as shown in Figure 2. In all of the studies, patients were instructed to begin with a lower dose and gradually increase it every day until they achieved pain relief, experienced side effects, or reached the maximum daily dose of 10 sprays. The investigation lasted two weeks. The majority of the studies investigated cannabis oil. The only studies that specified cannabis oral mucosal spray were those of Fallon M [21]and Lichtman [22]. Oil was studied in randomised trials by Zylla D, et al. [23] and Schloss J, et al. [25], but Bar-Lev Schleider, et al. [24]were the first to use inflorescence.
Withdrawal of Patients During the Study
A substantial number of patients in both the therapeutic and placebo groups were withdrawn in Fallon M, et al.’s study [21]. The mortality rates for therapy and placebo were 10 and 12.6% in trial 1 and 22 and 9% in study 2, respectively. In part B of trial 2, a disproportionately high number of deaths occurred in patients prescribed Sativex as opposed to a placebo. The Sativex group experienced more withdrawals than the placebo group. Adverse events are the primary cause of withdrawal rates. According to Lichtman et al., fifty-four (54) deaths from underlying cancer occurred during the research. The withdrawal rates matched those of Fallon M. et al.’s study 2 [21], while the fatality rates matched those of Fallon’s study 1. No patients have withdrawn from Zylla D et al.’s study because of adverse effects or other reasons from both groups (EC and DC). Each group had 1 patient die, which is the lowest number of any of our study comparisons [21,22,24,25]. Schloss J, et al. [25] reported eight deaths before and during the study, most of which were caused by their underlying cancer and other factors. Although the number of patients removed due to adverse events was not specified, some patients were removed due to a lack of compliance with the treatment. Bar-Lev Schleider [24], a large-scale study, reported 658 deaths. 290 patients withdrew from treatment, with pain relief being the most common reason (289.9%). 19.3% (56 patients) had side effects, indicating a small number of patients withdrawn due to side effects compared to the other four studies [21-23,25].
Pain Response
The main focus of this systematic review and all the clinical trials analysed was assessing the pain response of the patients who were medicated using different variations of medicinal cannabis. In five out of the six clinical trials, most patients experienced a reduction in pain as per the reported results for each. This was particularly evident in the trials conducted by Fallon M et al., “Study 1” and Lichtman, et al. [21,23], as statistically, both had significant P values of 0.274 and 0.0854, respectively. The specific route of administration may have played a role in reducing pain in these patients. In contrast, Nabiximols contain a combination of both THC and CBD (in a ratio of 1:1), which enhances the potency of this form of cannabinoid. In addition, utilising the oral mucosal route for administering treatment to these patients reduces the volume of THC lost in plasma when, in contrast, the formulation is inhaled or smoked [26].
Out of the six reported clinical trials presented in Table 1, only one, “Study 2” by Fallon M et al., resulted in patients experiencing an increased pain (NRS) score after ingesting medicinal cannabis. This result could have been due to other oncological-related symptoms, such as asthenia and anemia, impacting the patients’ responses to the efficacy of the Nabiximol oral mucosal spray or errors within the study’s experimental design. In the clinical trial conducted by Zylla D et al., patients were divided in half, with one group (the early cannabis group) receiving medicinal cannabis within the first three months. The other group (the delayed cannabis group) was given medicinal cannabis after a three-month incubation period of receiving routine oncology-related treatment. Even though all patients reported a significant reduction in pain, the study’s validity and reliability could be questioned. In particular, variables such as obtaining a small sample size (30), poor patient compliance with self-medicating/documenting personal activities, and a high dropout rate could reduce the credibility of this clinical trial, among all others [3].
Side Effects
Various side effects were reported across all studies, with nausea, vomiting, and dizziness being the most commonly documented. In both studies by Fallon M et al. and that of Lichtman et al., most patients receiving Nabiximol (as an oral mucosal spray) reported both nausea and vomiting after receiving treatment [21,23]. Such reported side effects are often seen in cannabinoid hyperemesis syndrome, a druginduced episode related to consuming cannabinoid-based products under all circumstances. This may explain why such side effects have been experienced by oncology patients utilising cannabinoid products in other trials surrounding pain management [27]. It is important to note that 2 of the clinical trials, those of which were conducted by Schloss J et al. and Zylla D et al., consisted of patients who experienced related psychological symptoms such as paranoia, euphoria, anxiety, and insomnia [3,25]. Specifically, in the study by Zylla D et al., 35% of the cohort were given medicinal cannabis via an inhaled formulation, with 8% experiencing anxiety and insomnia. Such a result can be scientifically explained as inhaled formulations of cannabis exposing patients to other combustion products within the medicine that enhance the potency of the administered drug. Thus, this theory could explain why certain patients are at a greater risk of experiencing specific types of adverse side effects [27].
Future Outcomes
With conflicting evidence due to limited quality studies conducted concerning the use of cannabinoids in the modulation of pain management in oncologic patients, researchers should develop clinical trial protocols which address the different stages of cancer and how cannabinoid treatments play a role or none, et al. [21]. Few trials have successfully recorded or analysed how the introduction of cannabis during earlier treatment stages of cancer symptoms can play a role in pain management [22] and should be investigated in further trials. Also, pharmacological modification of synthetics with cannabis infusion or concomitant use might prove beneficial in providing more significant pain relief in patients with further research.
Acknowledgement
We express our thanks to MAHMOUD Bassiony, Research assistant, Alexandria Faculty of Medicine, Egypt and Juan Carlos Ayala Alvarez for their help and support during this study.
Conflict of Interest
The authors declare no conflict of interest. There are no relevant financial or non-financial competing interests to report.
References
- Blake A, Wan BA, Malek L, DeAngelis C, Diaz P, et al. (2017) A selective review of medical cannabis in cancer pain management. Ann Palliat Med 6(Suppl 2): S215-S222.
- Wolf J, Urits I, Orhurhu V, Peck J, Orhurhu MS, et al. (2020) The role of the cannabinoid system in pain control: basic and clinical implications. Current Pain and Headache Reports 24(7): 35.
- Überall MA (2020) A review of scientific evidence for THC: CBD oromucosal spray (nabiximols) in the management of chronic pain. Journal of pain research 13: 399-410.
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, et al. (2005) Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310(5746): 329-332.
- Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE (2013) The role of endocannabinoids in pain modulation. Fundamental & clinical pharmacology 27(1): 64-80.
- Nikan M, Nabavi SM, Manayi A (2016) Ligands for cannabinoid receptors, promising anticancer agents. Life sciences 146: 124-130.
- Lu HC, Mackie K (2016) An introduction to the endogenous cannabinoid system. Biological psychiatry 79(7): 516-525.
- Sharon H, Brill S (2019) Cannabis-based medicines for chronic pain management: current and future prospects. Current Opinion in Anesthesiology 32(5): 623-628.
- Bennett M, Paice JA, Wallace M (2017) Pain and opioids in cancer care: benefits, risks, and alternatives. American Society of Clinical Oncology Educational Book 37: 705-713.
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, et al. (2008) μ-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. Journal of pharmacological sciences 108(3): 308-319.
- Fezza F, Bari M, Florio R, Talamonti E, Feole M, et al. (2014) Endocannabinoids, related compounds and their metabolic routes. Molecules 19(11): 17078-17106.
- Pertwee RG (2012) Targeting the endocannabinoid system with cannabinoid receptor agonists: pharmacological strategies and therapeutic possibilities. Philosophical Transactions of the Royal Society B: Biological Sciences 367(1607): 3353-3363.
- Lafaye G, Karila L, Blecha L, Benyamina A (2022) Cannabis, cannabinoids, and health. Dialogues in clinical neuroscience 19: 306: 316.
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, et al. (2015)Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 349(jan02 1): g764.
- PRISMA-P guidelines and protocol (nd) Equator Network.
- Cochrane Library (nd) Cochrane Library.
- Medline (nd) National Library of Medicine.
- Cochrane training (nd) Cochrane Training.
- International clinical trials registry platform search portal (2018, January 11) ICTRP Search Portal.
- Whiting P, Savović J, Higgins J (nd) ROBIS: Tool to assess risk of bias in systematic reviews Guidance on how to use ROBIS Media-Librar.
- Bar-Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, et al. (2018) Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. European journal of internal medicine 49: 37-43.
- Fallon MT, Albert Lux E, McQuade R, Rossetti S, Sanchez R, et al. (2017) Sativex oromucosal spray as adjunctive therapy in advanced cancer patients with chronic pain unalleviated by optimized opioid therapy: two double-blind, randomized, placebo-controlled phase 3 studies. British journal of pain 11(3): 119-133.
- Lichtman AH, Lux EA, McQuade R, Rossetti S, Sanchez R, et al. (2018) Results of a Double-Blind, Randomized, Placebo-Controlled Study of Nabiximols Oromucosal Spray as an Adjunctive Therapy in Advanced Cancer Patients with Chronic Uncontrolled Pain. Journal of pain and symptom management 55(2): 179-188e1.
- Schloss J, Lacey J, Sinclair J, Steel A, Sughrue M, et al. (2021) A Phase 2 Randomised Clinical Trial Assessing the Tolerability of Two Different Ratios of Medicinal Cannabis in Patients With High Grade Gliomas. Frontiers in oncology 11: 649555.
- Zylla DM, Eklund J, Gilmore G, Gavenda A, Guggisberg J, et al. (2021) A randomized trial of medical cannabis in patients with stage IV cancers to assess feasibility, dose requirements, impact on pain and opioid use, safety, and overall patient satisfaction. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 29(12): 7471-7478.
- Senderovich H, Waicus, S (2022) A case report on cannabinoid hyperemesis syndrome in palliative care: how good intentions can go wrong. Oncology research and treatment 45: 438-442.
- Urits I, Gress K, Charipova K, Habib K, Lee D, et al. (2020) Use of cannabidiol (CBD) for the treatment of chronic pain. Best practice & research Clinical anaesthesiology 34(3): 463-477.

 Research Article
Research Article