ABSTRACT
The world’s medical system has undergone a significant upheaval as a result of the catastrophic respiratory Coronavirus Disease (COVID-19), referred to as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). While its effects on respiratory symptoms are widely known, neurological manifestations have remained elusive. SARS-CoV-2 infects host cells by entering via angiotensin-converting enzyme-2 receptors (ACE-2), which are prominently overexpressed on intestinal epithelial cells, endothelial and smooth muscle cells of blood vessels, the heart, lung, renal tubular epithelial cells, and cerebrovascular endothelium. The pathophysiology of SAH in COVID-19 may include several pathways, including coagulopathy, cytokine storm, viral endotheliopathy, hypertension, and immunological regulation. Hemorrhagic cerebrovascular episodes are not uncommon in COVID-19, whether caused by an aneurysmal rupture or spontaneous subarachnoid hemorrhage (SAH). Bleeding into the area around the brain causes a kind of stroke known as subarachnoid hemorrhage (SAH). It is yet unclear what potential processes may be at play when SAH and SARS-CoV-2 are involved. SAH should be investigated in those who have significant neurological symptoms caused by this virus. This review analyses the case of a Caucasian woman who developed SAH in conjunction with COVID-19 infection. As a result, relevant literature and exploration of the putative pathogenetic processes are discussed for the correlation between SARS-Cov-2 and SAH.
Keywords: SARS-CoV-2; Coronavirus; COVID-19; Subarachnoid Hemorrhage; SAH; ACE-2 Receptors
Introduction
The novel coronavirus was reported in December 2019 during a pneumonia epidemic in Wuhan, China. It surged rapidly and the World Health Organization designated it a pandemic on March 11, 2020 [1]. COVID-19 was the term given to the disease induced by the virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Over 559 million confirmed cases and 6.3 million fatalities had been recorded globally as of July 17, 2022 [2]. SARSCoV- 2 infects host cells via Angiotensin Converting Enzyme-2 (ACE-2) receptors, ACE-2 is found on the apical membranes of polarized cells of the testis, cardiovascular epithelium, cardiac myocytes, cardiac fibroblasts, kidney, liver, intestine, brain, and lung epithelial cells, resulting in coronavirus disease (COVID-19)- related pneumonia as well as acute myocardial injury and long-term cardiovascular damage [3,4]. SARS-CoV-2 can potentially infect the nervous system, skeletal muscles, and respiratory tract. Neurologic involvement is increased in patients with severe infections, including acute cerebrovascular disorders, altered consciousness, and skeletal muscle damage [5]. The probable processes behind covid-19 involvement, stroke, and subarachnoid hemorrhage (SAH) are yet unknown. However, according to numerous studies, covid-19 can increase intracranial pressure, increasing the likelihood of an intracranial aneurysm [6]. At the moment, there seems to be minimal information available on the SAH following COVID-19. An overt or covert link between the incidence of spontaneous SAH and COVID-19 has yet to be established. It is crucial to identify parameters for the diagnosis and therapy of patients with COVID-19 infection and SAH. We herein report a case of a non-comorbid young woman infected with SARS-CoV-2 presenting with severe cough and headache and eventually saccular aneurysmal SAH, who recovered with conservative management and a literature review exploring links between SAH and SARS-CoV-2.
Case Report
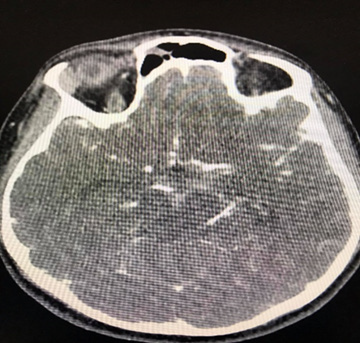
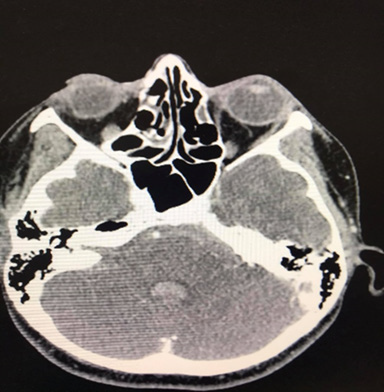
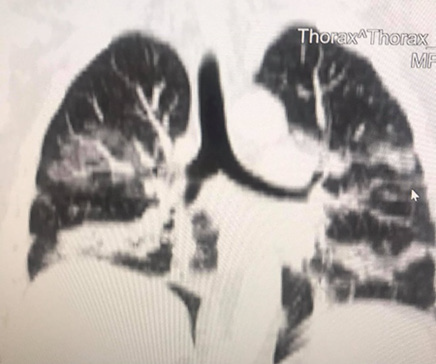
A 35-year-old Caucasian woman, 178cm, 72kg, presented with nausea, vomiting, dry cough, fever, anosmia, and headache for 5 days. Comorbidities or prior neurological episodes were not reported. The patient was alert and oriented upon admission to the emergency room. The patient denied any family history of brain aneurysms or past medical history of any neurological deficits. The likelihood of infection by the novel coronavirus was raised after reports of cough, fever, and anosmia in the preceding 5 days. As a result, throat swab samples were collected for 2019-CoV RNA RT-PCR, which confirmed SARS-CoV-2 infection. The patient was admitted to ICU to receive hospital care. Within 48 hours of hospital admission, the patient was disoriented and confused and developed acute respiratory failure. After clinical stabilization, a cranial CT scan was performed, and a Fisher score II SAH was visualized (Figures 1 & 2). The patient was placed on ventilation and had suffered a subarachnoid intraventricular hemorrhage. Upon further observation, the dynamic condition did not improve according to neuroimaging, later the patient’s consciousness was suppressed, and her Glasgow Coma Scale (GCS) score was 3. The patient was transferred to Caucasus Medical Center, Evex hospitals, Tbilisi, Georgia, to receive specialist care. Upon admission, the patient was in a comatose state, while her photoreaction and corneal reflexes were sluggish. The patient underwent further testing and a chest CT scan was performed and it demonstrated bilateral glass opacities, suggestive of COVID-19 pneumonia (Figure 3).
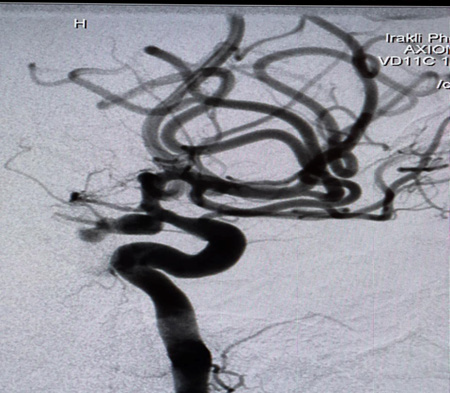
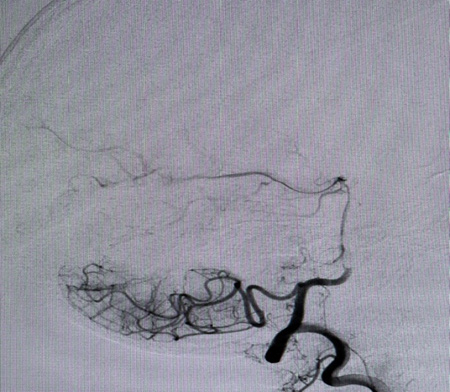
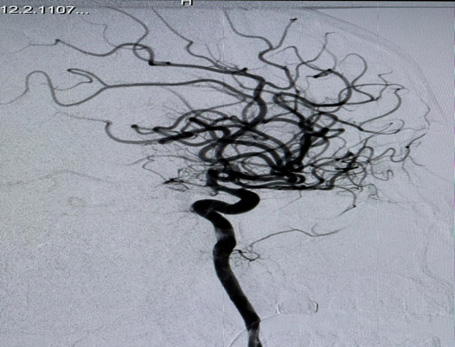
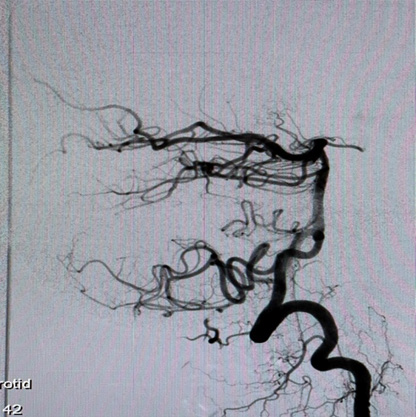
A Selective digital cerebral angiography was performed - an aneurysm of a complex configuration, a two-chamber, left posterior cerebral artery aneurysm, with a wide neck saccular aneurysm was detected. A spasm of the basilar artery was also detected as shown in Figures 4 & 5. Based on the angioarchitecture of the aneurysm, and to prevent its re- rupture, endovascular closure was performed via balloon-assisted embolization, and complete occlusion of the aneurysm was achieved. Pharmaco-angioplasty for correction of pronounced vasospasm was performed using Percutaneous Transluminal Angioplasty (PTA) of the basilar artery using Nimodipine I/A infusion (Figures 6 & 7). Based on the angioarchitecture of the aneurysm, and to prevent its rerupture, endovascular closure was performed via balloon-assisted embolization, and complete occlusion of the aneurysm was achieved. Pharmaco-angioplasty for correction of pronounced vasospasm was performed using Percutaneous Transluminal Angioplasty (PTA) of the basilar artery using Nimodipine I/A infusion (Figures 6 & 7). The post-operative period proceeded with positive dynamics, without complications. The patient became more active and the headaches decreased. Neurologically at discharge, the patient experienced clear consciousness, adequate orientation in time and environment, and relatively fewer headaches. The patient was discharged after 24 days, with no neurological disabilities. The patient was advised to rest for 2-3 weeks and begin neurorehabilitation.
Figure 3: A chest CT scan in coronal section reveals ground-glass opacities, indicating lung infection by the new coronavirus
Epidemiology
Subarachnoid hemorrhage was the 53rd leading cause of death worldwide in 2019, with 11.2 million cases and 373,000 fatalities and a gross mortality rate of 3.33 percent. Furthermore, 240,000 deaths in patients aged over 60 was noted, compared to 103,000 deaths from patients aged between 40 to 59 [7]. Following the study conducted by Sepide Kashefiolasl et al, 10 dated March 21st, 2022, it discussed the prevalence of COVID-19 and Subarachnoid Hemorrhage concerning the age of the patients. In their study, they retrospectively analyzed some subsamples of patients that independently suffered from COVID-19 and SAH and the other group of patients that shared the homogeneity of both diseases. To make their conclusion more referable they underlined the prepandemic era as well. It was then thereby found that there were 12 out of 56 (21%) patients aged less than 50 with a diagnosis of aSAH whereas in comparison to the pre-covid era there were 19 out of 84 (23%) patients aged less than 50 with a diagnosis of aSAH. Concerning this data there was a conclusion that SAH interlinked with COVID-19 was much more prevalent in patients of young age, to emphasize more on the findings they used multivariable analysis to verify significant factors for a favorable outcome (mRS ≤ 0–2) after aSAH during the COVID pandemic [8]. In another similar study conducted by Qureshi AL et al, 282,718 patients were evaluated to assess the risk of SAH development in patients with COVID-19. It was concluded that 0.3% to 1.2% of the patients develop Sub arachnoid Hemorrhage as a complication of Covid-19 [9]. In a gender-based study of the incidence of SAH in association with Covid, male patients were found to be younger than older female patients. The evident causes of younger age group predominance were cigarette smoking in males and hypercholesterolemia in females [10].
Pathophysiology
SARS-COV-2 is a member of the coronavirus family, namely the Beta coronavirus. Beta coronaviruses are known to frequently infiltrate the central nervous system. This behavior has also been observed in other coronaviruses such as SARS, MERS as well as porcine hemagglutinating encephalomyelitis [11]. SARS-COV-2 infects host cells by entering via angiotensin-converting enzyme-2 receptors (ACE-2). ACE-2 receptors are omnipresent within the human body, particularly overexpressed on intestinal epithelial cells, endothelial and smooth cells of blood vessels, heart, lung, renal tubular epithelial cells [12], and in cerebrovascular endothelium which interacts with the viral “s” protein (homotrimer spike glycoprotein) [13]. A potential risk factor that causes intracranial hemorrhage (ICH) is intracerebral capillaries bursting as a result of direct endothelial toxicity. Additionally, endothelial damage may initiate a series of events involving the coagulation cascade, the complement system, and proinflammatory cytokines. Ultimately, this causes increased permeability, breakdown of cellular tight junctions, and the disruption of the blood-brain barrier leading to ICH. In addition, the renin-angiotensin-aldosterone pathway is disrupted by SARS-Cov-2 suppressing the expression of the ACE- 2 receptor. As a result, the autoregulation of cerebral blood flow is compromised, and the local endothelium accumulates high amounts of angiotensin II. This subsequently results in uncontrolled hypertension, significantly raising the risk of subarachnoid hemorrhage (SAH) and ICH [13].
In microvascular lesions of cerebral hemorrhage, increased D-dimer level enhances fibrinolytic function and plasmin generation, which may result in the hypercoagulable conditions. Elevated D-dimer levels are associated with a hyper-fibrinolytic, pro-inflammatory state in SARS-Cov-2 patients, and these levels have been shown to correlate with the severity of the illness and may raise the risk of intracranial bleeding [14]. Innate and acquired immune responses that are elicited by the virus are the main defense mechanisms that protect the host organisms from the crippling effects of SARS-Cov-2 infections. Identification of pathogen-associated molecular patterns (PAMPs) and induction of antigen-specific adaptive immunity is of prime importance to cause these responses. The body’s response to viral infections is influenced by the secretion of cytokines, chemokines, leukotrienes, proteases, reactive oxygen species, and the rate of viral clearance [15]. Furthermore, SARS-Cov-2 causes systemic inflammation as a result of cytokine down-regulation leading to septic shock [16]. Inflammation associated with SARS-CoV-2 and aneurysmal rupture is both heavily influenced by macrophages. The generation of IL- 1, IL-6, and tumor necrosis factors by macrophages have a role in SARS-CoV-2 infection and cerebral aneurysm rupture [17]. Studies have shown males had higher rates of severe Sars-Cov-2 symptoms necessitating ICU admission and worse ischemic stroke outcomes upon hospital release while females had higher rates of milder symptoms [18].
Comorbidities Associated with Covid
Patients with Subarachnoid hemorrhage plus SARS-COV2 had a greater chance of mortality than patients with subarachnoid hemorrhage without SARS-COV2 or patients with SARS-COV2 without subarachnoid hemorrhage. These individuals had a higher incidence of systemic comorbidities which served as a factor in the elevated risk. Patients with COVID-19 and subarachnoid hemorrhage had higher rates of pneumonia, pulmonary embolism, urinary tract infection, acute kidney damage, liver failure, Heart failure, acute MI, septic shock, and respiratory failure [9]. A study evaluating Patients hospitalized with COVID-19 disease reports a significant and high risk of thrombotic events including stroke and SAH. 52% of Covid -19 patients present SAH, while 95% of them have a history of hypertension and 60% have a history of diabetes Mellitus, 40% have cardio embolism and 5% have vessel disease, and 35% cryptogenic [19]. Hypertension (HTN) and chronic heart disease were the two most common vascular comorbidities, as documented in various studies on ICH in COVID-19 patients [13]. Due to the resulting autonomic imbalance, more individuals are developing cardiac arrhythmias as the coronavirus 2019 pandemic advances relentlessly. The most frequent rhythm disturbance in COVID-19 infection patients is sinus tachycardia, which can be brought on by fever, hypoxia, and hemodynamic compromise. Transient sinus bradycardia is also a potential sign of COVID-19, according to a retrospective series of 4 patients. Numerous ventricular arrhythmias can happen as a result of triggers such as COVID-19 infection-induced systemic inflammation and severe respiratory insufficiency. Additionally, many medications used to treat COVID-19 infection have the potential to affect the cardiac system by lengthening the QT interval and inducing polymorphic Ventricular Tachycardia (VT) in the form of Torsade de pointes (TdP) [20].
A hemorrhage was found in a group of covid 19 patients 67 years old where the majority of comorbidities diseases were hypertension, diabetes, and obesity. SARS-CoV-2 may raise blood pressure and increase the risk of cerebral hemorrhage once it enters the bloodstream, especially in people who already have high blood pressure [21]. When compared to controls, patients with spontaneous ICH or SAH and concurrent COVID infection were more likely to be members of racial or ethnic minorities, diabetics, and obese, and they also had higher mortality rates and longer hospital lengths of stay [22]. Diabetic patients were more likely to be admitted to the intensive care unit (ICU) during the SARS epidemic at a rate that was higher than that of non-diabetic patients. Research and Reviews proved that diabetic patients have a significantly lower absolute lymphocyte count than those who do not have diabetes. Clinical studies revealed that once diabetic individuals contracted SARS-CoV2 due to a variety of causes such as decreased physical activity and irregular nutrition, the insulin dose rose and blood glucose control became challenging. Additionally, as the virus employs the human angiotensin-converting enzyme (ACE2) as a receptor for cellular entrance, which enhances personto- person transmission, the increased infectivity and virulence of SARS-CoV2 in diabetes is explained. Noting that the reninangiotensin system (RAS) family, which has been linked to diabetes, contains the key component ACE2 [23].
Additionally, a review of the international Health Outcome Predictive Evaluation for SARS-COV2 registry evaluating the effect of renal function on admission and mortality with SARS-CoV-2 infection found that 30% of patients had kidney dysfunction upon admission and that CKD was prevalent in 8.5 % of infected patients [24]. In COVID-19, AKI is linked to a greater risk of death. A case in point is sepsis. Microvascular dysfunction, a rise in vascular permeability, and tissue damage are its defining characteristics. Cardiomyopathy, viral myocarditis, and left ventricular failure are additional conditions that can cause hemodynamic abnormalities. Additionally, cytotoxic reactions harm podocytes and tubules and cause hematuria, proteinuria, and AKI. Moreover, collapsing glomerulopathy is an extra consequence that is brought on by a direct viral effect, the presence of increased cytokines from the systemic inflammatory response, or both [25].
Risk Factors for Covid and SAH
Risk factors of high-rate mortality are based on a cohort study, Elevated INR, severe pulmonary symptoms, and spontaneous hemorrhagic presentation [26]. Cigarette smoking, cocaine use, hypertension, low body mass index, first-degree relatives with hemorrhagic stroke, caffeine in pharmaceutical products, lower educational attainment, and nicotine in pharmaceutical products are all risk factors for SAH that can be modified. Age, sex, and race are further risk variables that cannot be changed [27]. To be more specific hypertension can classify as the first and the most notable risk factor caused by SAH in human beings regardless of age and sex which’s followed by the second risk factor in young males is cigarette smoking and hypercholesterolemia in the older woman [10]. There is a strong association between a higher risk of Intracranial Aneurysm (IA) and aneurysmal Subarachnoid Hemorrhage (aSAH) and genetic propensity to smoking, sleeplessness, and high blood pressure. The risk of IA and aSAH may also be influenced by factors like physical activity, body mass index, triglyceride levels, and lowdensity lipoprotein cholesterol levels. These findings support the triangulation of information regarding IA and aSAH risk factors and call for additional research in future extensive MR and other epidemiological studies. The biggest risk factors for IA and aSAH, according to the current MR study, include smoking and high blood pressure. Additionally, this study discovered data that suggests insomnia may be a brand-new risk factor for IA and aSAH [28].
In the indirect way of SAH hemodynamic stress, chronic inflammation and vascular wall remodeling are the most common causes of the ruptured Cerebral aneurysm which leads by the reactive oxygen species through the activation of nuclear factor Kappa-B to the endothelial dysfunction. Hemodynamic stress is brought on by the activation of the renin-angiotensin system, which causes hypertension. Unlimited consumption of (alcohol, antioxidants vitamins, B vitamins, flavonoids, and n-3 fatty acids) leads to increased blood pressure which increases the risk of the rupture of cerebral aneurysm and causes SAH [29]. The most important risk factors of SAH based on studies are Smoking, hypertension, and excessive alcohol consumption, Besides, there are many other uncertain factors such as nonwhite ethnicity, HRT, hypercholesterolemia, and diabetes in the etiology [30,31]. Cigarette smoking and hypertension increase the risk of cerebrovascular diseases which are the main causes of SAH. However, cigarette smoking cessation doesn’t decrease this risk [32].
Regarding covid 19, The Elderly COVID-19 patients, who had a higher risk of hospitalization, mechanical ventilation, and mortality, demonstrated dyspnea as a risk factor. Additionally, because of weakened immunity, patients with cancer and hematologic malignancies are more susceptible to SARS-CoV-2 infection. An Individual may be more susceptible to COVID-19 infection if they are pregnant in addition to these risk factors. This may be due to physiological changes in the immune system and placental immaturity during the first trimester [33]. One of the most important complications of Covid 19 disease is thrombosis, while preventing this thrombotic complication by using antithrombotic agents, there are important side effects, from the use of these agents, such as hemorrhagic stroke, subarachnoid hemorrhage, and intracranial bleeding [34].
Complications
Major Complication Associated with SARS-CoV-19
Pneumonia: The Lower respiratory tract’s innate and acquired defense systems produce an inflammatory response after infection, as in the case of SARS-CoV-19. The cytokines TNF-a, IL-8, and IL-1 released by the resident alveolar macrophage in the lungs attract inflammatory cells like neutrophils to the parenchyma thereby generating clinically symptomatic pneumonia [35,36]. Additionally, the macrophages function as Antigen-presenting T-cells (APC)s, activating a plethora of immune responses, cell-mediated, humoral, complement-activated, and antibody-production. As a result, the lung parenchyma becomes inflamed, and capillary leakage leads to fluid-filled alveolar sacs, thus producing the fundamental pathogenesis of pneumonia [36]. Patients experience productive cough (greenish, yellow, or bloody mucus), tachycardia, tachypnea, fever with chills, malaise, loss of appetite, and myalgia. A fraction experience altered mental status, abdominal pain, stabbing chest pain and further systemic findings. Physical examination common findings include crackles, dullness on percussion and egophony and tactile fremitus (both suggestive of consolidation) [35,36].
Acute Respiratory Distress Syndrome (ARDS)
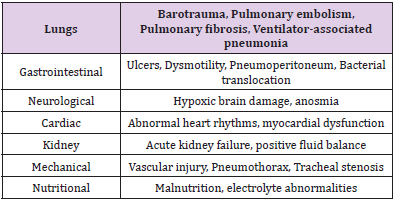
ARDS is a cause of respiratory failure in critically ill patients and is the acute onset of pulmonary edema (noncardiogenic), atelectasis, hypoxemia and the need of mechanical ventilation. ARDS is marked by increased permeability to fluid and protein across the lung endothelium leading to edema in the lung interstitium. Damaged tight junctions between type I and II alveoli, the edematous fluid translocate into the alveoli space. Typical hallmarks of ARDS include increased permeability to fluid, proteins, neutrophils and RBCs, leading to their accumulation within alveolar space [37]. Vascular hypoxemia seen is caused by ventilation-to-perfusion disbalance as well as right-to-left intrapulmonary shunting. Furthermore, hindered carbon dioxide excretion is a major component of respiratory failure, resulting in increased pulmonary dead space with elevated ventilation [37]. Increasing pulmonary dead space and decreasing respiratory compliance are predictors of ARDS mortality [37]. Diffuse alveolar damage (DAD) is the main pathologic finding and is characterized by the destruction of both alveolar type I and II cells. This ultimately leads to pulmonary edema as the destroyed alveolar type II cannot remove leaked fluid, this then is followed by hyaline membrane depository on the alveolar walls. During this time, surfactant produced by alveolar type II cells cannot reduce surface tension and inflate the alveoli for respiration. During the recovery process, collagen deposited may not be completely reabsorbed, limiting alveolar expansion and thus respiration [37,38]. A plethora of problems arises from ARDS which can be seen in the Table 1 below [37,38].
Table 1: Further systemic complications of ARDS. It can be seen that ARDS in its own right is a complication, yet it can bring additional problems that can ultimately lead to death.
Multi-Organ Failure and Death
Whilst common cold symptoms are observed in mild forms of COVID-19. In severe cases, there is the involvement of different organs that can quite frequently lead to death, often characterized by acute lung failure, liver failure, kidney injury and cardiovascular disease, and a spectrum of hematological and neurological abnormalities [39,40]. It is suggested that the main cause of multi-organ failure is the cytokine storm, which is induced by inflammatory mediators, endothelial dysfunction, coagulation abnormalities, and inflammatory cell organ infiltration [40]. Along with the plethora of complications previously mentioned, death can ultimately occur and can be a result of multi-organ failure or any other complication such as ARDS or Acute heart failure [39,40].
Common Complications Pertaining SAH and SARS-CoV-19.
In comparison to COVID-19, some patients with the condition: SAH (Subarachnoid Hemorrhage) will survive and make a satisfactory recovery. However, a significant number of individuals still do develop serious complications which further decline their health and can potentially lead to death. Complications can be classified into intracranial or extracranial where, according to Hall and O’Kane (2018), intracranial consequences are factored in the initial treatment while the latter is closely monitored [41]. Furthermore, according to Daniere et al., complications can also vary in severity, from acute, subacute, and chronic listing the following in respective order: hydrocephalus, vasospasms, and cognitive disorders [42]. In many instances, the complications can be closely related to the activation of the sympathetic nervous system, catecholamines, and the systemic inflammatory response syndrome (SIRs) (Garg and Bar, 2017) [43]. Data supported by Hammer et al., (2020), has shown that the following are statistically significant complications found after SAH: pneumonia, sepsis, hydrocephalus, and delayed cerebral ischemia [44]. Apart from the neurological complications seen in SAH, a patient may also develop secondary non-neurological conditions, such as neurocardiogenic injury, pulmonary edema, and hyperglycemia (Chen, et al. [45]). From these non-neurological conditions, it is common to observe cardiac and pulmonary manifestations following the complication of SAH [38].
Conclusion
This case study and literature analysis on COVID-19 and SAH established that patients with a prior COVID-19 infection have a higher likelihood of developing SAH as opposed to patients who have not acquired COVID-19. The primary etiology of SAH during COVID-19 infection was proven to be thrombotic vascular events, which caused microvascular lesions of cerebral hemorrhage and disrupted the angioarchitecture. Additionally, these lesions were most frequently seen in patients with a history of comorbidities like diabetes mellitus and hypertension resulting in higher mortality rates. Regardless of the patient’s age or comorbidities, the ratio of major respiratory complications such as pneumonia and ARDS were significantly higher. The major determinants of higher mortality in patients with SAH and COVID-19 were elevated INR, insidious pulmonary presentations (effusion, ARDS, pneumonia), and spontaneous hemorrhagic conditions. It was further noted that the strokes were typically initiated after the COVID-19- associated lung infection had completely subsided in the body. Furthermore, this led to coagulation in the arteriovenous system of the brain, resulting in the individual becoming more prone to brain hemorrhages. Although there were generalized reviews stating that COVID-19 infection caused an increase in the intracranial pressure while simultaneously surging the risk of aneurysms, there was ambiguity in the data that determined the underlying mechanism of the aforementioned. Consequently, thorough research in this area would be beneficial in determining factual data.
Funding
None.
Conflicts of Interest
The authors declare no conflict of interest.
References
- (2020) WHO Director-General's opening remarks at the media briefing on COVID-19.
- (2022) Weekly epidemiological update on COVID-19.
- Zheng YY, Ma YT, Zhang JY, Xie X (2020) COVID-19 and the cardiovascular system. Nature reviews. Cardiology 17(5): 259-260.
- Yang F, Zhao H, Liu H, Wu X, Li Y (2021) Manifestations and mechanisms of central nervous system damage caused by SARS-CoV-2. Brain research bulletin 177: 155-163.
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA neurology 77(6): 683-690.
- Cezar-Junior AB, Faquini IV, Silva J, De Carvalho Junior EV, Lemos L, et al. (2020) Subarachnoid hemorrhage and COVID-19: Association or coincidence?. Medicine 99(51): e23862.
- (2019) Subarachnoid Hemorrhage-Level 4 cause | Institute for Health Metrics and Evaluation.
- Kashefiolasl S, Qasem LE, Brawanski N, Moritz Funke, Fee Keil, et al. (2022) Impact of COVID-19 Pandemic on Treatment Management and Clinical Outcome of Aneurysmal Subarachnoid Hemorrhage - A Single-Center Experience. Front Neurol 13: 836422.
- Qureshi AI, Baskett WI, Huang W, Shyu D, Myers D, et al. (2021) Subarachnoid Hemorrhage and COVID-19: An Analysis of 282,718 Patients. World neurosurgery 151: e615-e620.
- Inagawa T (2005) Risk factors for aneurysmal subarachnoid hemorrhage in patients in Izumo City, Japan. Journal of neurosurgery 102(1): 60-67.
- Steardo L, Steardo L, Zorec R, Verkhratsky A (2020) Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta physiologica (Oxford, England) 229(3): e13473.
- Shirbhate E, Pandey J, Patel VK, Kamal M, Jawaid T, et al. (2021) Understanding the role of ACE-2 receptor in pathogenesis of COVID-19 disease: a potential approach for therapeutic intervention. Pharmacological reports PR 73(6): 1539-1550.
- Abbas R, El Naamani K, Sweid A, Schaefer JW, Bekelis K, et al. (2021) Intracranial Hemorrhage in Patients with Coronavirus Disease 2019 (COVID-19): A Case Series. World neurosurgery 154: e473-e480.
- Mercado ES, Diaz JB (2021) Spontaneous Non-Aneurysmal Subarachnoid Hemorrhage as Initial Presentation of COVID-19: A Case Report. Medical Reports & Case Studies 6(5): 1-4.
- Karnik M, Beeraka NM, Uthaiah CA, Nataraj SM, Bettadapura A, et al. (2021) A Review on SARS-CoV-2-Induced Neuroinflammation, Neurodevelopmental Complications, and Recent Updates on the Vaccine Development. Molecular neurobiology 58(9): 4535-4563.
- Daly SR, Nguyen AV, Zhang Y, Feng D, Huang JH (2021) The relationship between COVID-19 infection and intracranial hemorrhage: A systematic review. Brain hemorrhages 2(4): 141-150.
- Dodd WS, Jabbour PM, Sweid A, Tjoumakaris S, Gooch MR, et al. (2021). Aneurysmal Subarachnoid Hemorrhage in Patients with Coronavirus Disease 2019 (COVID-19): A Case Series. World neurosurgery 153: e259-e264.
- Trifan G, Goldenberg FD, Caprio FZ, Biller J, Schneck M, et al. (2020) Characteristics of a Diverse Cohort of Stroke Patients with SARS-CoV-2 and Outcome by Sex. Journal of stroke and cerebrovascular diseases: the official journal of National Stroke Association 29(11): 105314.
- Rothstein A, Oldridge O, Schwennesen H, Do D, Cucchiara BL (2020) Acute Cerebrovascular Events in Hospitalized COVID-19 Patients. Stroke 51(9): e219-e222.
- Manolis AS, Manolis AA, Manolis TA, Apostolopoulos EJ, Papatheou D (2020) COVID-19 infection and cardiac arrhythmias. Trends in cardiovascular medicine 30(8): 451-460.
- Santana MF, Frank C, Almeida T, Jeronimo C, De Araújo Pinto, et al. (2021) Hemorrhagic and thrombotic manifestations in the central nervous system in COVID-19: A large observational study in the Brazilian Amazon with a complete autopsy series. PloS one 16(9): e0255950.
- Ravindra VM, Grandhi R, Delic A, Hohmann S, Shippey E, et al. (2021) Impact of COVID-19 on the hospitalization, treatment, and outcomes of intracerebral and subarachnoid hemorrhage in the United States. PloS one 16(4): e0248728.
- Zhou Y, Chi J, Lv W, Wang Y (2021) Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes/metabolism research and reviews 37(2): e3377.
- Uribarri A, Núñez-Gil IJ, Aparisi A, Becerra-Muñoz VM, Feltes G, et al. (2020) Impact of renal function on admission in COVID-19 patients: an analysis of the international HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) Registry. Journal of nephrology 33(4): 737-745.
- Hassanein M, Radhakrishnan Y, Sedor J, Vachharajani T, Vachharajani VT, et al. (2020) COVID-19 and the kidney. Cleveland Clinic journal of medicine 87(10): 619-631.
- Altschul DJ, Unda SR, De La Garza Ramos R, Zampolin R, Benton J, et al. (2020) Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clinical neurology and neurosurgery 198: 106112.
- Broderick JP, Viscoli CM, Brott T, Kernan WN, Brass LM, et al. (2003). Major risk factors for aneurysmal subarachnoid hemorrhage in the young are modifiable. Stroke 34(6): 1375-1381.
- Karhunen V, Bakker MK, Ruigrok YM, Gill D, Larsson SC (2021) Modifiable Risk Factors for Intracranial Aneurysm and Aneurysmal Subarachnoid Hemorrhage: A Mendelian Randomization Study. Journal of the American Heart Association 10(22): e022277.
- Czekajło A (2019). Role of diet-related factors in cerebral aneurysm formation and rupture. Roczniki Panstwowego Zakladu Higieny 70(2): 119-126.
- Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, et al. (2005) Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 36(12): 2773-2780.
- Teunissen LL, Rinkel GJ, Algra A, Van Gijn J (1996). Risk factors for subarachnoid hemorrhage: a systematic review. Stroke 27(3): 544-549.
- Yao X, Zhang K, Bian J, Chen G (2016) Alcohol consumption and risk of subarachnoid hemorrhage: A meta-analysis of 14 observational studies. Biomedical reports 5(4): 428-436.
- Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, et al. (2021) Risk factors for severe and critically ill COVID-19 patients: A review. Allergy 76(2): 428-455.
- Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, et al. (2021) Anticoagulation use and Hemorrhagic Stroke in SARS-CoV-2 Patients Treated at a New York Healthcare System. Neurocritical care 34(3): 748-759.
- (2019) Symptoms and Diagnosis.Lung.org.
- Jain V, Vashisht R, Yilmaz G, Bhardwaj A (2022) Pneumonia Pathology. In StatPearls. StatPearls Publishing.
- Sweeney RM, McAuley DF (2016) Acute respiratory distress syndrome. Lancet (London, England) 388(10058): 2416-2430.
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, et al. (2019) Acute respiratory distress syndrome. Nature reviews. Disease primers 5(1): 18.
- Acharya Y, Alameer A, Calpin G, Alkhattab M, Sultan S (2022) A comprehensive review of vascular complications in COVID-19. Journal of thrombosis and thrombolysis 53(3): 586-593.
- Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, et al. (2020). Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: The REPROGRAM Consortium Position Paper. Frontiers in immunology 11: 1648.
- Hall A, O'Kane R (2018) The Extracranial Consequences of Subarachnoid Hemorrhage. World neurosurgery 109: 381-392.
- Danière F, Gascou G, Menjot de Champfleur N, Machi P, Leboucq N, et al. (2015) Complications and follow up of subarachnoid hemorrhages. Diagnostic and interventional imaging 96(7-8): 677-686.
- Garg R, Bar B (2017) Systemic Complications Following Aneurysmal Subarachnoid Hemorrhage. Current neurology and neuroscience reports 17(1): 7.
- Hammer A, Ranaie G, Erbguth F, Matthias Hohenhaus, Martin Wenzl, et al. (2020) Impact of Complications and Comorbidities on the Intensive Care Length of Stay after Aneurysmal Subarachnoid Haemorrhage. Sci Rep 10: 6228.
- Sheng Chen, Qian Li, Haijian Wu, Paul R Krafft, Zhen Wang, et al. (2014) The Harmful Effects of Subarachnoid Hemorrhage on Extracerebral Organs. BioMed Research International.

 Review Article
Review Article