Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ephrem Shimelis*, Fanos Tadesse, Shahbaz Bashir and Jan Paeshuyse
Received: August 11, 2022; Published: August 19, 2022
*Corresponding author: Ephrem Shimelis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Department of Microbiology, Immunology and Veterinary Public Health, Ethiopia
DOI: 10.26717/BJSTR.2022.45.007253
The IgY immunoglobulin originated from chicken egg yolk plays a significant roles in diagnosis and vaccine production for viral, bacterial and assess the occurrence of parasites in animals, however, its usage as an alternative source to a mammalian antibody production that causing stress, scare, injure to serum collector and bleeding during blood collection, expensive price using mammals, and low antibody concentration in comparation with egg yolk; so that the using in medical and veterinary was limited. The aim of these experimental training was to extract, purify and in vitro challenging of IgY of commercial chicken eggs with the Newcastle disease in order to produce chicken egg yolk antibody against the Foot-and-Mouth disease in Ethiopia that may use for the development’s diagnostic kits and vaccine production. From the six-chicken egg yolk purchased from supermarket, the IgY was extracted by polyethylene glycol (600mg), its protein concentration was measured by 280nm spectrophotometer and the resulted the protein content ranged from 2.45624 to 3.635 in mg/ ml. the purity of the extracted IgY were measured by 10%SDS-PAGE and the reactivity against the Newcastle disease were obtained at 19.72-39.53 and 60.53-67.57 kDa bands respectively representing light and heavy chains. The neutralization ability of the extracted IgY was also determined by hemagglutination inhibition test by using RBC collected from the chickens and the result revealed that the antibody titer of yolk was different significantly (P<0.05) in the collected eggs in which the range of HI-titers in Reciprocal of Log2 was 3 to 8. The experiment performed was given a promise that the chicken can be an alternative to the mammalian antibody production for developing diagnostic kit and vaccine production without causing the bleeding, stress and injured the animal from which serum can be collected.
Keywords: Antibody; Chicken; Egg Yolk and Igy Immunoglobulin
Abbreviations:: CEE: Chicken Embryonated Eggs; HA: Hemagglutination; HI: Hemagglutination Inhibition; Ig: Immunoglobulin; NCD: Newcastle Disease; PAGE: Polyacrylamide Gel Electrophoresis; PEG: Polyethylene Glycol; RBC: Red Blood Cell; SDS: Sodium Dodecyl Sulfate
The antibody produced by an infected or vaccinated host animals plays a significant role in post-exposure protection of similar disease, developments of diagnostic materials and production vaccine for prevention and controls of animals and human disease [1,2]. In most of the developing country, the commercially supplied antibody (immunoglobulin) play critical roles in developments of diagnostic assays, therapy, and purification of specific target compounds in animals and human research and diagnosis [3]. Traditional polyclonal antibodies production uses large animals such as horses, sheep, pigs and also rabbits and guinea pigs while monoclonal antibodies production required mice and rats’ spleen. Nowadays, most of the research and diagnostic laboratory uses rabbits and mice for polyclonal and monoclonal antibody production, respectively [4]. Recently, the eggs laid by animals of different species, including birds, plays an important role in production of immunoglobulin IgY that is the most abundant in chicken egg yolk [5,6]. IgY that produced only in bird immune system is consistently secreted in bloods and transferred to the egg-yolk trans ovarially [7,8].
Functionally, immunoglobulin Y (IgY) in avian is equivalent to immunoglobulin G (IgG) in mammalian species [9]. the hens IgG is called IgY (yolk immunoglobulin), which can enter the egg and protect the fetus until it hatches [10]. Structurally, IgY has two heavy chains and two light chains (68 kDa and 27 kDa, respectively) and the Absence of the Hinge region in its molecular structure make similar to mammalian IgE [11,12]. When we compare with mammalian IgG, IgY produced in chicken egg yolk have many advantages antibody production including: the noninvasiveness , large-scale production (∼40 g IgY/y/hen), high epitope specificity and affinity to mammalian antigen due to the phylogenetic distance between chickens and mammals, inability to interact with rheumatoid factors, bacterial fragment crystallizable (Fc) receptors, or activate mammalian Complement and recognize more epitopes of the highly conserved mammalian proteins than other mammalian IgGs [5,13]. This antibody can be stored in the egg yolk at 4 °C for at least a year. IgY is resistant to heat and acid [10].
Currently, The interest for immunoglobulin Y (IgY) isolated from the whole eggs or yolks have been used in veterinary medicine as an inexpensive immunoglobulin Y source [14] that can be used as alternative for antibody production because it reduces stress to animals such as horses sheep, pigs and also rabbits, guinea pigs, mice and rats for the production of antibodies bleeding, scarify, and expensive methods [12,15] as IgG immunoglobulins of one species are immunogenic for other species [10], however, in Ethiopia production of polyclonal and monoclonal antibody by using the layer hens was not adapted in research and diagnostic laboratory. Therefore, the experimental training on extraction, purification and in vitro challenging with Newcastle disease virus of egg yolk IgY immunoglobulin was designed by keet project at HPI laboratory of KU-Leuven University faculty of Bioscience Engineering, according to the collaborative agreement with the Addis Ababa University. The aim of the training programme was to give the training at a KU-Leuven and produced the IgY immunoglobulin against the Footand- Mouth disease in Ethiopia by using the layer hens as input for the development of inexpensive diagnostic kit and vaccine in Ethiopia.
An experimental training was conducted from October 2019 up to November 2019 G.C in KU Leuven, faculty of Bioscience Engineering on extraction, purification, detection and in vitro challenging of chicken egg yolk IgY immunoglobulin with of field Newcastle. Purposively, six [13] eggs were purchased from the supermarket and IgY was extracted by PEG-600mg precipitation. The protein concentration of the purified egg yolk was calculated according to the Lambert-Beer law with an extinction coefficient of 1.33 for IgY after measured photometrically at 280 nm [16]. Detection and evaluation of antibody titer against NCDV were carried out by SDS PAGE analysis, western blotting, and Hem agglutination test.
Preparation of Viral Antigen: According to WHO. (2013) [17] one virulent NDV field isolates was used in this study. NDV isolates was propagated by inoculation into allantoic cavity of 9- to 10-day-old anti- NDV antibody-free CEEs obtained from HPI. The inoculated CEEs were monitored for 24-72 h to observe embryo death. Allantoic fluid of inoculated CEEs was harvested and centrifuged at 1000×g, for 30 min. The supernatant was collected and tested for the presence of NDV with hemagglutination (HA) and HI test. Infective allantoic fluid-containing NDV was clarified by centrifugation at 1500×g for 10 min at 4°C, filtered with 0.4μm filter and kept in micro-tubes at −80°C for further use.
IgY Extraction and Purification: The Polyethylene Glycol (PEG 600mg) precipitation method for IgY purification was adapted according to Pauly, et al. [6]. Briefly, Once the fresh eggs were cleaned with 75% alcohol and cotton balls, the eggshell was cracked carefully and the egg yolk were transferred to a filter paper to remove the remaining egg white, the egg yolk skin membrane was cut with lancet, and the egg yolk was transferred 50 ml Falcon tube. Then, after the volume of the egg yolk was increased by twice of its volume PBS (Phosphate buffered saline), 3.5% of total volume PEG (600mg) of the total volume was added to the solution and mixed by vortexed, rolled for 10 min by a roller. before the tubes were centrifuged at 10,000 rpm for 20 min at 4oC. The supernatant was then poured through a folded filter paper and the same procedure of centrifugation was followed with the 8.5% PEG 6000 and the pellet was dissolved by using 1 ml PBS that was raised to 10ml by adding 9 ml PBS. The solutions were then mixed with 12% PEG 6000 and the tube centrifuged at 10,000 rpm for 20 min at 4oC. The supernatants were discarded, and the pellet dissolved in 800 μl PBS using a glass stick and vortex, the extracts were transferred to a dialysis membrane (Cellu Sep H1, part #5050-28) to remove the salt.
IgY Confirmation by SDS-PAGE and Western Blot: The molecular weight of chicken IgY antibodies purified by using Polyethylene Glycol (PEG 6000) precipitation was measured by 10 % SDS-PAGE gel electrophoreses in which the A broad range protein standard (Bio-Rad 161-0317) from 4.46 kDa to 97.2 kDa was used as the marker and its specificity to Newcastle disease virus (NCDV) determined by Western blot according to Gao, et al. [18]. Briefly, the extracted and purified egg yolk sample was loaded over 10% polyacrylamide discontinuous gels under reducing condition, and its bands were separated by running on constant voltage of 180 V and stained by Coomassie Blue stain. To conduct the western blot, carefully, the six samples loaded on the 10 % SDS-PAGE gel electrophoreses was washed with double distilled water. Then, Then, the Proteins were also blotted on a polyvinylidene difluoride (PVDF) membrane with a Mini Trans- Blot® Electrophoretic Transfer Cell (instruments settings: 100 V and 350 mA, Bio-Rad, Hercules and blocked with 5% skimmed milk at room temperature for 90 min. The membrane was washed for five times with Tris buffered saline containing 0.05% Tween 20 (TBST). The membranes were incubated with primary antibodies overnight on a shaking incubator at 4˚C. again, the membranes were washed five times with TBS and incubated with alkaline phosphatase conjugated secondary antibody Antichicken IgY (IgG) (whole molecule) Sigma A9171) at a diluted at 1:16,000 with blocking buffer for 90 min on a shaker. Finally, the membranes were washed four times with TBST (5 min for each) and detected by autoradiography after the membrane was stained by BCIP_/ NBT (Sigma/B5655) alkaline phosphates substrate for 5 min and washed in water to stop the reaction.
Hemagglutination Inhibition Test (HI Test): The serum HAI antibody titer is often used as a surrogate marker of protection and is an important immunogenicity measure in humans [19]. According to WHO. (2013) [17], Briefly, 25μL of PBS per well was dispensed in clear 96-wells of V-bottom plates, then 50 μL of IgY were placed on the first wells of each row of except wells of antigen and RBC controls and 2-fold dilutions of 25 μL of the IgY suspension were made across the entire plate by using multichannel pippets. Then, 25 μL of diluted virus containing 4 hemagglutination units (HAU) of virus was added to each well except wells of RBC controls and incubated for 30 min at room temperature, then, 50 μl 0.5% erythrocyte suspension were added to each well and shake/agitate the plate(s) to mix thoroughly and incubate at room temperature for 10–30 minutes. Finally, Hemagglutination inhibition endpoint (the highest dilution of IgY causes complete inhibition of viral hemagglutination) were scored and recorded as reciprocal log2 values of the highest dilution of HI. The titer of hemagglutinationinhibiting antibodies was the highest serum dilution that inhibited the agglutination of 75% −100% of red blood cells and expressed as the reciprocal of that dilution
SDS-PAGE and western blotting analysis were done by Lab Image 1D Application Version: 7.1.3, kapelan. labimage. 2001- 2019 Kapelan Bio-Imaging GmbH, http://www.kapelanbio.com/. HI analysis was conducted according to (Stephenson, et al. [15]) in which initial dilution did not give a positive response, the titer was recorded as less than the reciprocal initial dilution. So, the of resporical value Log2 =2 were used as standard for interpretation of IgY concentrations because our initial dilution was 1:2. The statistical difference between the six eggs were analyzed by regression and One Sample t-test on excel-2019 in which a P-value of less than 0.05 was considered to indicate statistical significance.
Protein concentration (mg/ml) measured at 280 nm (1:50 diluted with PBS) and calculated according to the Lambert-Beer law with an extinction coefficient of 1.33 for IgY of different eggs (n= 6). The results indicated the protein concentration ranged from 2.45624 to 3.635 in mg/ml (Table 1).
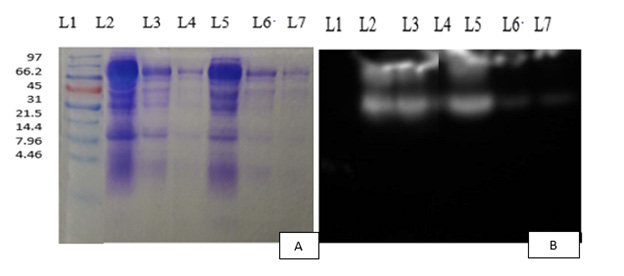
The result of the water-soluble fractions obtained during IgY extraction were analyzed by SDS-PAGE Sodium dodecyl sulfate polyacrylamide gel electrophoresis with the purity of. 19.72-39.53 and 60.53-67.57 kDa respectively representing light and heavy chains (Figure 1a) that detected by SDS-PAGE that there was statistical difference between each egg used in our experiment (P-Value< 0.05) (Table 2). The reactivity of the extracted IgY was carried out by using Western blot analysis in which anti-Newcastle disease virus, extracted IgY was used as the first antibody and using HRP-rabbit anti-chicken IgY as the second antibody results obtained demonstrated that viral antigen in our experiment was recognized by extracted IgY. The results obtained from the Western blot showed two protein bands corresponding to the heavy and light chains of each chicken egg IgY (Figure 1b) which indicates that the purified protein from the egg yolk was the chicken IgY.
Note: L1: Lane of standard ladder, L1: lane of egg yolk (IgY-1), L2: lane of egg yolk (IgY-2), L3: lane of egg yolk (IgY-3), L4: lane of egg yolk (IgY-4), L5: lane of egg yolk (IgY-5), L6: lane of egg yolk (IgY-6)
Figure 1:
a) 10% SDS-PAGE profile of anti-NCD IgY antibodies. The two IgY chains appeared resolving SDS-PAGE gel.
b) Western blot identification of IgY using HRP conjugated rabbit anti-chicken IgY heavy and light. Remaining bands might represent other antibodies or protein fragments of unknown origin (n = 6).
The results of the present study revealed that the ability of the extracted IgY immunoglobulin inhibit the Newcastle disease virus were ranged from 3 to 8 HI-titers in resporical of Log2 (Figure 2). The antibody titer of eggs yolks was significantly different between the egg yolks (P<0.05) (Table 3).
Vaccinated chickens were response to the only structured protein as the is produced from the structured proteins [20]. The antibodies purified from the serum of vaccinated mammals plays an important role in scientific research and diagnosis, however, it can cause stress and bleeding to the mammals from which the serum can be collected. Currently, the chicken egg yolk antibody production is recommended as a best alternative that allows and kept them under almost all their natural conditions [3]. In poultry the evaluation antibodies in the chicken eggs are important for detecting the vertical transfer of antibodies against the specific disease and checking the effectiveness of vaccine administered to the chicken for prevention and controls of the targeted poultry disease [21]. So that the aim of these experimental training was to purify and detected the IgY against the Newcastle disease viruses from the six chicken eggs purchased from the supermarket in Leuven and the proposed experiment done at KU-Leuven HPI laboratory at during the training time schedule prepared by Prof. Jan Paesuyshe under KEET projects during October, 2019 to November, 2019. The experiment was conducted on commercial chicken eggs based on the report of Marcq, et al. [22] that explain the IgY production period in the layer hens’ egg can be extended up to a two years because an interruption during the lay or the practice of molting hens before or after initiating antibody production has little or no impact on the collected level of IgY.
The IgY was extracted by the Polyethylene Glycol (PEG 6000) precipitation method from the egg yolk, the extract was dialyzed overnight in 0.1 % saline (1,600 ml) and the protein content (mg/mL) was measured photometrically at 280 nm (1:50 diluted with PBS) and calculated according to the Lambert-Beer law. The results indicated the protein concentration (mg/ml) ranged from 2.45624 to 3.635 in mg/ml (Table 1) as it was measured by the 280nm spectrophotometer. The obtained result was agreed with that reported by Ahmad, et al. [3] antibody concentrations of 3.66 mg/ml for Single Comb White Leghorns egg yolk and 3.476 mg/ml reported by Putri, et al. [23]. The purity of yolk IgY was established in PAGE by applying reducing conditions and the specific to chicken IgY against an epitope on the heavy and light chain constant region was confirmed by Western blotting. Figures 1a & 1b shows the SDSPAGE and WB results, respectively. The results show that there were IgY antibodies against the NCD from chicken eggs as the band of egg yolk IgY purified by 10% SDS-PAGE obtained in range of 60.52 kDa to 67.58 kDa heavy chain and 16.09 kDa to 39.53 kDa light chain (Figure 1a) was specifically reacted with the NCD antigen used for the experiment as it detected by western blot (Figure 1b).
However, the result obtained was greater than the 25,6 kDa result of Pandu et al. [24] indicated the expressed of recombinant F protein by SDS-PAGE and Western blot; It agrees with the 67 kDa F protein result of Shahid et al. [25] and Abdolmaleki et al. [10]. Western blot analysis demonstrating the expression of F and HN protein in transgenic plants and less than the result reported by Javad et al. [23]. The soluble proteins (100 μg) were run on 10% SDS-PAGE and subjected to immunoblotting analysis with polyclonal HN- and F-specific antibodies of approximately 75 kDa corresponding to the HN-F glycoprotein. Also, the result was agreed with the result reported by Sudjarwo, et al. [26] on the Purification and characterization protein of anti-dengue specific immunoglobulin Y for diagnostic kit of dengue. Santos et al. [27] reported that there were Various bands with different molecular weights, varying from 220-25 kDa in which heavy chain of IgY weighed 68 kDa and the light chain 27 kDa, the visualized accessory protein bands represented the impurities that were not fully eliminated in the isolation process, justifying a purification process by thiophilic adsorption. the IgY light chain from some egg yolks may stain lightly with Coomassie Blue. Using the extinction coefficient for IgY the yield in milligrams of protein can be calculated. Occasionally the IgY is incompletely reduced, and this can result in several other bands of IgY. The Western blot further illustrates that the isolated protein is mostly IgY [28].
Challenging of the extracted IgY with the NCD antigen indicated that there was a neutralizing antibody response activity against the NCD virus; when it was determined by Hemagglutination inhibition test that is still the most alternatively used serological method for measuring anti- NDV antibody levels in poultry sera. However, there is the probability of the false positives result occurrence in tested of sera [29]. The results of the present experiment revealed that the antibody(IgY) titers extracted by Polyethylene Glycol (PEG 6000) from the egg yolks were different significantly (P<0.05) in the collected eggs in which the range of HI-titers in Resporical of Log2 was 3 to 8 with the mean of 5.5.The result was greater than 3.66 antibody extracted by ammonium sulfate and less than 10.8 extracted by chloroform that was reported by Ghaniei et al. [8]. However, the result mean of HI was 5.5, the result reported by Al-attar, et al. [30]. indicated the higher HI titer against NDV was 4.625 when layer chickens were vaccinated with live attenuated Newcastle disease vaccine with three booster doses. However, the day post vaccination of chicken that laid our experimental eggs was not known since it directly purchased from the supermarket, the obtained 5.68 (28/30) HI result was agreed with the result reported at 10.6 Average age of the tested chickens in months. reported by Neuhaus & Lierz [31].
The results indicated somewhat difference in IgY concentration, band of purified protein and in vitro neutralizing activities due to composition of the vaccine, adjuvant, amount of antigen, nutrition effects, egg yolk weight, the age of the birds, genetic variations [22,32]. The difference in the neutralizing response may occur between different eggs originated from different individual chickens due to simultaneously change the F protein cleavage site from a mono-basic to a poly-basic motif, which likely would have the effect of increasing replication, spread, and immunogenicity [33]. in vaccinated animals, antibody production can be affected by several factors including immunogenicity, antigen quality and quantity, antigen form and solubility, animal species, and immunization route [1]. The variation in percentage of transfer throughout the experiment may be attributed to several factors as reported by Coakley et al. and Nahla, et al. [34].
Our results revealed that the IgY can be purified from the chicken egg yolk in non-invasiveness methods with good protein band reacted with the Newcastle disease virus, high titer and good neutralization efficiency against ND viruses that promised us that it can be produce antibody against different animal disease by using the layer hens us a laboratory animals since the IgY produced in chicken egg yolk; can reduce bleeding to mammals, only eggs are needed following immunization and low quantities of antigen are required to obtain high and long-lasting IgY titers in the yolk of immunized hen eggs. The benefits of IgY technology and its universal application in both research and medicine is expected to expand at a largescale. It is expected that IgY will play an increasing role in research, diagnosis, and immunotherapy in the future. From the above conclusion we can forwarded the following recommendation:
1) The egg should be collected from the experimentally vaccinated chicken, rather than using commercial eggs.
2) The experiment should repeat and confirmed by giving enough time
3) The extracted and purified IgY should challenge with many heterogenic field NCD viruses.
4) Further study should be conducted on purification and challenging of IgY.
5) The study should extend to diagnostic kit development and in vivo challenging of the IgY.
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been checked by the authors. Since the egg used in our experiments were infertile that daily supplied for consumer consumption, the ethical clearance regarding animal was not needed.
The specific thanks of authors go to the all staffs and students of KU-Leuven university, faculty of Bioscience Engineering for their arranging the building block training for me to develop the costeffective and simple method for production of polyclonal antibody by using the layer hen egg-yolk that can be input for diagnostic kits and vaccine for FMDV in Ethiopia. Specially, I acknowledge Prof. Jan Paeshuyse who inviting me trained at the institution by arranging all materials, financial and technical support during my training.
The authors declare that there is no conflict of interests.


