Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Zhicheng Huang1, Aimee L Hong2, Jian Chen3, Xi Chen1, Lei Yang1, Hang Li4* and Zhiqiang Sun3*
Received: July 07, 2022; Published: August 24, 2022
*Corresponding author: Hang Li, Department of Thoracic Oncology, Jilin Province Cancer Hospital, 1018 lake road, Changchun, Jilin, China
Zhiqiang Sun, MD, Department of Interventional Radiology, Jilin Province Cancer Hospital, Changchun, Jilin, China 1018 lake road, Changchun, Jilin, China
DOI: 10.26717/BJSTR.2022.45.007255
Background and Methods: N6-methyladenosine (m6A) is the most prevalent and abundant post-transcriptional RNA modification in eukaryotic mRNA. YTHDFs, as m6A readers, destabilize m6A-containing mRNAs and play an important role in the tumorigenesis and metastasis of multiple malignancies. However, knowledge regarding the YTHDF homology in connection to non-small cell lung cancer (NSCLC) is still inconclusive. This study systematically analyzed the expression profiles and prognostic values of the YTHDF family in lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) patients, by using ONCOMINE, UALCAN, Kaplan-Meier Plotter, cBioPortal, GePIA 2.0 and Network analyst databases.
Results: The mRNA of the YTHDF family was over-expressed in both LUAD and LUSC. Lower YTHDF1 (HR 0.48, 95% CI:0.38-0.61, P=1e-9), YTHDF2 (HR 0.47, 95% CI: 0.37-0.6, P=3.3e-10), YTHDF3 (HR 0.44, 95% CI: 0.35-0.56, P=1.1e-11) mRNA expression was significantly associated with worse overall survival (OS) in the LUAD patients, but not in LUSC patients. The expressions of YTHDF1,2,3 were highly mutated in LUAD patients, with YTHDF1, YTHDF2, and YTHDF3 gene mutation rates being 36%, 19%, and 32%, respectively. In addition, the prognostic value of YTHDFs in the different clinicopathological features based on intrinsic subclasses and treatments of LUAD patients was further assessed in the KM plotter database.
Conclusion: Our studies elucidate the expression and prognostic role of YTHDFs in NSCLC. Our results indicated that mRNA expression of YTHDF1, YTHDF2, YTHDF3 is a potential predictor of outcomes for LUAD patients, but not in LUSC patients. These data suggest that YTHDFs would be potential prognostic biomarkers and novel therapeutic targets for LUAD patients.
Keywords: YTHDF; m6A RNA; LUAD; NSCLC; Prognostic Role
Lung cancer (LC) is the leading cause of all cancer-induced deaths worldwide [1,2]. In all cases of lung cancer, non-small cell lung cancer (NSCLC) is responsible for a majority measure of approximately 84% [2]. NSCLC can be subdivided into 3 types: lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC), and large cell carcinoma (LCC) [3]. LUAD is the most common form of NSCLC, found in smokers and nonsmokers alike, as well as being found more commonly in women than in men and in younger generations under the age of 45 [4]. It compensates for around 50% of all NSCLCs while the next most common type, LUSC, is responsible for about 30% of cases [5]. LUSC begins its replication in the squamous cells that line the large airways of the lung and holds the strongest connection to smoking out of all the types of NSCLC [6]. The focus of our study will pertain to LUAD and LUSC exclusively. mRNA modifications play a critical role in diverse biological processes including cancer development and progression [7-9]. Several mRNA modifications have been identified, including N1-methyladenosine (m1A), N6-methyladenosine (m6A), 5-methylcytidine (m5C), and the 5′ cap modifications N 6,2-O-dimethyladenosine (m6Am) and 2′-O-methylation (2′-O-me) [10]. Among these, m6A is the bestcharacterized and most prevalent mRNA modification [10,11]. The process of m6A mRNA modification is found to be both dynamic and reversible through the work of methyltransferases (m6A writers), RNA binding proteins (m6A readers) and demethylases (m6A erasers) [11]. A 20 gene catalog of proteins primarily functioning as regulators of m6A methylation has been identified and curated, broken down into eleven readers, seven writers, and two erasers [8,11,12]. Consequently, m6A is recognized by the YT521-B homology (YTH) domain family of proteins which directs and utilizes different complexes for the purpose of regulating RNA signaling pathways such as RNA metabolism, RNA splicing, RNA folding, and protein translation [13,14]. YTH N6-Methyladenosine RNA Binding Proteins include three members (YTHDF1-3) in human tissues [14]. YTHDF1-3, containing the YTH domain, have been characterized as direct m6A readers and function together to mediate the degradation of m6A mRNAs [14,15]. Widespread genetic abnormalities in regulators, such as mutations and copy number variations, have been found across cancer types as emerging evidence continues to be compiled. This association has concluded a link to tumor proliferation, invasion, differentiation, tumorigenesis, and metastasis and functions as oncogenes or antioncogenes in malignant tumors. So far, knowledge regarding the reconstitutes of m6A, particularly the reader YTHDFs in lung cancer, is still lacking. In this study, we aimed to systematically characterize the molecular alterations and clinical relevance of YTHDF1, YTHDF2, and YTHDF3 between LUAD and LUSC patients.
This study was approved by the Ethics Committee of the Jilin province cancer hospital. We retrieved the original data from the online databases and analyzed them by using the online databases.
The mRNA expression of YTHDFs members in different types of cancer and related adjacent control tissues were analyzed by using the ONCOMINE database [16] (www.oncomine.org). Data of the mRNA expression in these tissues were analyzed by students’ t-test, with p value < 0.01, fold change > 1.5 and gene rank as 10%.
We used UALCAN database (http://ualcan.path.uab.edu/), a free online open-access platform with gene expression data sets from TCGA database [17], analyzed the relative mRNA transcriptional levels of YTHDFs genes of interest between normal and different stages of cancer tissues. Data was analyzed by students’ t-test and p <0.01 was recognized as statistically significant.
We used the Kaplan-Meier Plotter database (KM Plotter, http://kmplot.com/analysis/), an online database with different gene expression statuses and survival information of lung cancer patients and analyzed the prognostic values of YTHDFs members [18]. The mean expression of gene was used to split the mRNA level of each YTHDFs genes. The prognostic value of YTHDFs in lung cancer patients were analyzed and compared between the high expression cohort and low expression cohort.
We analyzed mutations in the genomic profiles of YTHDFs family members by using the cBioportal database with the z-score threshold ±1.8 [19,20]. We also collected the 40 frequently altered neighbor genes of each YTHDFs member in LUAD patients by using similar gene detection functions on the Gepia2.0 database [21] (http://gepia2.cancer-pku.cn/#index). These frequently altered neighbor genes were pooled with YTHDFs members and applied for protein interaction networks by using the Networkanalyst platform [22].
We applied 120 genes significantly associated with these mutations for GO and KEGG enrichment analysis via Metascape database with min overlap at 3, p-value cut off at 0.05, and min enrichment at 3 (https://metascape.org/gp/index.html#/main/ step1) [23]. These genes were administrated to GO enrichment analysis for biological processes (BP), cellular components (CC), molecular functions (MF) and KEGG pathway analysis individually.
The mRNA expression of genes in lung cancer tissues was analyzed by Student’s t-test. Kaplan-Meier survival plots were generated with survival curves compared using the log-rank test. P values less than 0.05 was considered as statistically different [24].
To explore the distinct prognostic and potential therapeutic value of different YTHDF members in patients with cancer, the mRNA expression was analyzed by the Oncomine database (www. oncomine.org). As shown in Figure 1, mRNA expressions of 3 YTHDF family members in 20 types of cancers were first measured and compared to normal tissues by the Oncomine database. 4 times more cases were reported that mRNA expressions of the YTHDF1, 2 and 3 family are higher in these 20 types of cancer tissues compared to related normal tissues (Figure 1). However, the expression of YTHDF family in lung cancer has not been well studied. Thus, we compared the different transcriptional levels of YTHDFs family members in the two major type of NSCLC, LUAD and LUSC, by using the UALCAN database. As shown in (Figure 2), the mRNA expressions of YTHDF1, YTHDF2, and YTDHF3 were significantly higher in both LUAD (Figure 2) and LUSC (Figure 2) tissues compared to adjacent normal lung tissues (P<0.001). We then further analyzed the relationship of transcriptional levels of the YTHDF family and the 4-clinical stages in both LUAD and LUSC patients. As shown in Figure 3, mRNA of YTHDF1, YTHDF2, and YTHDF3 were found higher in at least one of LUAD (Figures 3A-3C) and LUSC’s (Figure 3) clinical stages (P<0.001). No mRNA expression differences of YTHDF1, YTHDF2, and YTHDF3 were detected between the earlier stages 1, 2, and the later stages 3 and 4 in both LUAD and LUSC patients (Figure 3). These data suggested that the expression of YTDHFs is strongly correlated to tumor initiation but not to tumor progression.
Figure 1: Transcriptional expression of YTHDFs in 20 different types of cancer diseases (ONCOMINE database). The difference in transcriptional expression was compared by students’ t-test. Cut-off of p-value and fold change were as following: p value: 0.05, fold change: 1.5, gene rank: 10%, data type: mRNA.
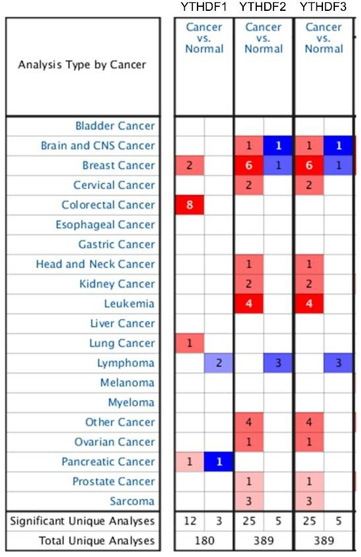
Figure 2: mRNA expression of distinct YTHDFs family members in LUAD, LUSC tissues and adjacent normal lung tissues (UALCAN). (A-C) mRNA expression of YTHDF1 (A), YTHDF2 (B) and YTHDF3 (C) in LUAD tissues and adjacent normal lung tissues. (D-F) mRNA expression of YTHDF1 (D), YTHDF2 (E) and YTHDF3 (F) in LUSC tissues and adjacent normal lung tissues. *** p<0.001.
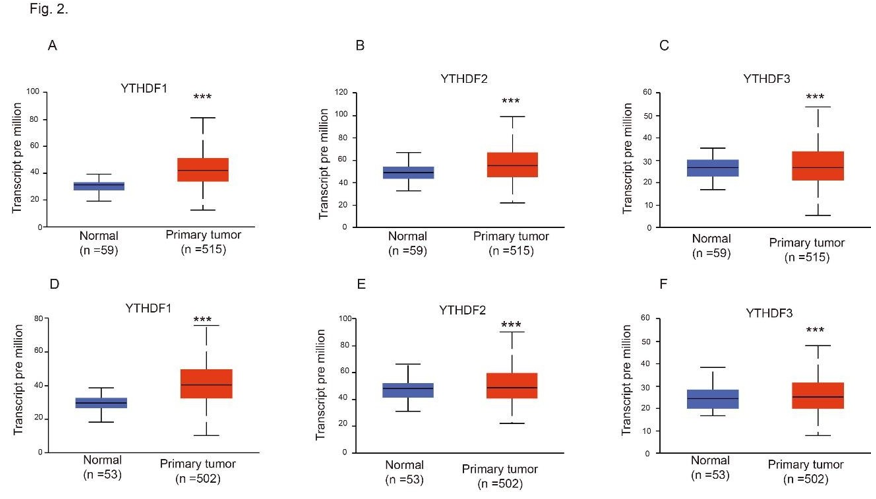
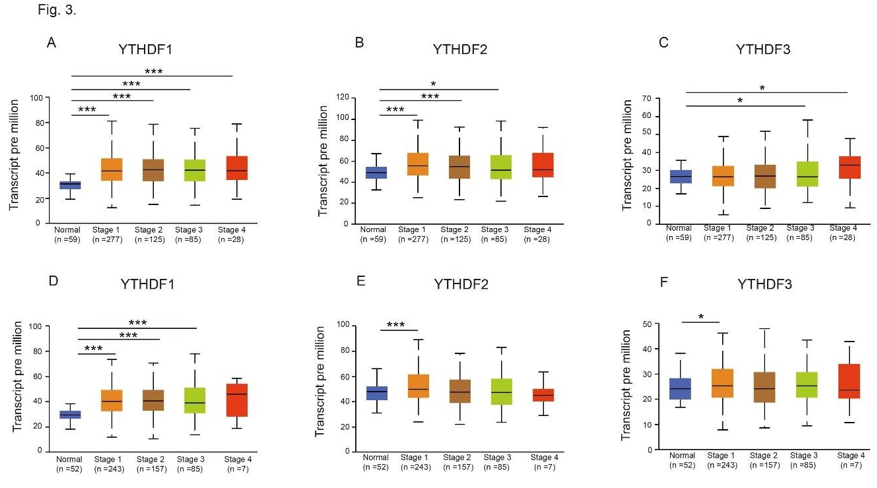
Figure 3: Relationship between mRNA expression of distinct YTHDFs family members and individual cancer stages of LUAD and LUSC patients. (A-C) mRNA expression of YTHDF1 (A), YTHDF2 (B) and YTHDF3 (C) in individual cancer stages of LUAD and adjacent normal lung tissues. (D-F) mRNA expression of YTHDF1 (D), YTHDF2 (E) and YTHDF3 (F) in individual cancer stages of LUSC and adjacent normal lung tissues. *p<0.05, ***p<0.001.
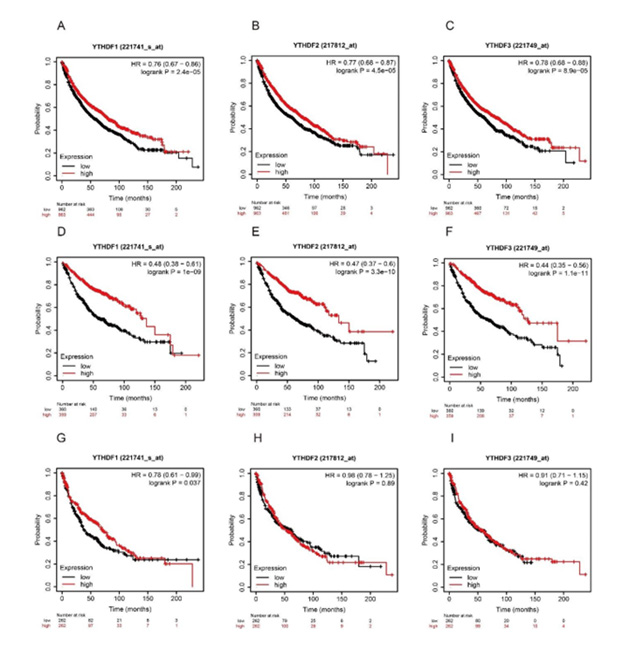
Next, we analyzed the prognostic values of mRNA expressions of distinct YTHDF family members in the overall lung cancer patients (Figure 4), in the LUAD patients (Figure 4), and in the LUSC patients (Figure 4) by using the Kaplan-Meier Plotter. As was shown in (Figure 4), lower YTHDF1 (HR 0.76, 95% CI: 0.67- 0.86, P=2.4e-05), YTHDF2 (HR 0.77, 95% CI: 0.68-0.87, P=4.5e-05), YTHDF3 (HR 0.78, 95% CI: 0.68-0.88, P=8.9e-05) mRNA expression was significantly associated with shorter OS in lung cancer. We found a similar correlation of the YTHDF family mRNA expressions with the OS of LUAD patients, but not in LUSC patients. As was shown in (Figure 4), lower YTHDF1 (HR 0.48, 95% CI:0.38-0.61, P=1e-9), YTHDF2 (HR 0.47, 95% CI: 0.37-0.6, P=3.3e-10), YTHDF3 (HR 0.44, 95% CI: 0.35-0.56, P=1.1e-11) mRNA expression was significantly associated with worse OS in the LUAD patients. On the other hand, YTHDF1, YTHDF2, YTHDF3 mRNA expressions were not correlated with the prognosis of LUSC cancer patients (Figure 4). This data suggested that the mRNA expression of YTHDFs would be a potential prognostic biomarker to LUAD patients but not for LUSC patients.
Figure 4: Relationship between mRNA expression of distinct YTHDFs family members and individual cancer stages of LUAD and LUSC patients. (A-C) mRNA expression of YTHDF1 (A), YTHDF2 (B) and YTHDF3 (C) in individual cancer stages of LUAD and adjacent normal lung tissues. (D-F) mRNA expression of YTHDF1 (D), YTHDF2 (E) and YTHDF3 (F) in individual cancer stages of LUSC and adjacent normal lung tissues. *p<0.05, ***p<0.001.
To further analyze the association of YTHDFs mRNA expression with various LUAD subclasses, we detected the survival effects of YTHDFs in different smoking status, different genders and different clinical stages. As shown in (Table 1), the high expression of YTHDF2 was correlated to better OS in LUAD patients both with and without smoking, and the high expression of YTHDF3 was correlated with better OS in LUAD patients with smoking. While the expression of YTHDF1 was not associated with the OS in LUAD patients with different smoking statuses. The high expression of YTHDF1/2/3 were associated with better OS both in female and male LUAD patients (Table 1). In addition to the LUAD patients from different clinical stages, the high expression of YTHDF1 or 2 were strongly correlated to better OS of LUAD patients in stage 1 and 2 but not in stage 3 (Table 1). The high expression of YTHDF3 predicts better OS in LUAD patients from stage 1, 2 and 3. Thus, these results suggested the roles of YTHDFs as potential prognostic predictors in LUAD patients with different subclasses.
Next, we also checked the prognostic effects of YTHDFs in LUAD patients with different treatments, including without chemotherapy, with chemotherapy and with surgical margins negative. As shown in (Table 2), high expression of YTHDF1 was significantly correlated with better OS in LUAD patients with chemotherapy, and high expression of YTHDF3 was strongly correlated with better OS in LUAD patients both with and without chemotherapy. However, the expression of YTHDF1 or YTHDF3 was not correlated to the OS in LUAD patients with surgical margins negative. Interestingly, the high expression of YTHDF2 was strongly correlated with better OS in LUAD patients with surgical margins negative, but not correlated with the LUAD patients with and without chemotherapy (Table 2).
We further investigated the genetic alteration in YTHDFs in LUAD patients. As shown in Figure 5A, a high mutation rate (87%) of YTHDFs was observed in LUAD patients (cBioPortal). YTHDF1, YTHDF2, and YTHDF3 genes display high genetic alterations with mutation rates being 36%, 19%, and 32%, respectively. After analyzing the genetic alterations in YTHDF s and their prognostic value in LUAD patients, we also collected the 40 frequently altered neighbor genes of each YTHDFs member in LUAD patients by using similar gene detection functions on the Gepia2.0 database (http:// gepia2.cancer-pku.cn/#index). Further, we analyzed predicted functions and pathways of the mutations in YTHDFs and their 120 frequently altered neighbor genes in LUAD patients by using the Networkanalyst platform. We constructed a network of YTHDF mutations and their 120 frequently altered neighbor genes (Figure 5).
Figure 5: Genetic mutations in YTHDFs in LUAD patients (cBioPortal) (A). High mutation rate (87%) of YTHDFs was observed in LUAD patients. YTHDF1, YTHDF2 and YTHDF3 genes display high genetic alterations with mutation rates as 36%, 19% and 32%, respectively (A). Predicted functions and pathways of the mutations in YTHDFs and their 120 frequently altered neighbor genes in LUAD patients (Gepia 2.0). The regulation of mRNA metabolic process genes including UBC, MTOR, YWHAZ, KHDRBS1, PSMA7, HNRNPU and HNRNPK were significantly related to YTHDFs mutations. The network of YTHDFs mutations and their 120 frequently altered neighbor genes was constructed by using the Networkanalyst database (B). GO functional enrichment analysis predicted.
three main functions of YTHDFs mutations and their 120 frequently altered neighbor genes (Metascape database), including biological process, cellular components and molecular functions (C-E). KEGG pathway analysis (Metascape database) on YTHDFs and their 120 most frequently altered neighbor genes (F).
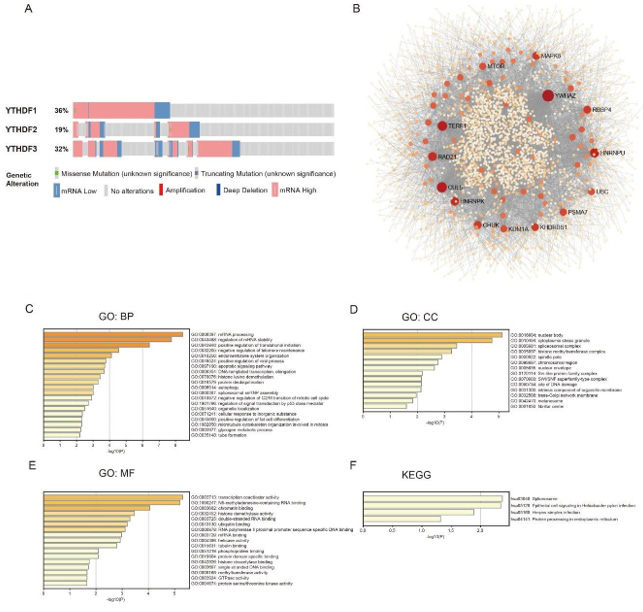
As was shown in (Figure 5), the regulation of mRNA metabolicprocess genes, including UBC, MTOR, YWHAZ, KHDRBS1, PSMA7, HNRNPU and HNRNPK were significantly related to YTHDFs mutations. Moreover, we processed the GO functional enrichment analysis for YTHDFs mutations and their 120 frequently altered neighbor genes, including biological processes, cellular components, and molecular functions (Figure 5). As shown in (Figure 5), biological processes such as GO:0043488 (regulation of mRNA stability), GO:0045948 (positive regulation of translational initiation),GO:0032205 (negative regulation of telomere maintenance), GO:0010256 (endomembrane system organization),GO:0048524 (positive regulation of viral process),GO:0097190 (apoptotic signaling pathway),GO:0006354 (DNA-templated transcription, elongation),GO:0070076 (histone lysine demethylation),GO:0016579 (protein deubiquitination),GO:0006914 (autophagy),GO:0000387 (spliceosomal snRNP assembly),GO:0010972 (negative regulation of G2/M transition of mitotic cell cycle), GO:1901796 (regulation of signal transduction by p53 class mediator), GO:0051640 (organelle localization),GO:0071241(cellular response to inorganic substance),GO:0045600 (positive regulation of fat cell differentiation), GO:1902850 (microtubule cytoskeleton organization involved in mitosis), and GO:0005977(glycogen metabolic process),GO:0035148 (tube formation) were remarkably regulated by the YTHDF mutations in LUAD patients.
Cellular components including GO:0016604 (nuclear body),GO:0010494 (cytoplasmic stress granule), GO:0005681 (spliceosomal complex), GO:0035097 (histone methyltransferase complex), GO:0000922 (spindle pole), GO:0098687 (chromosomal region),GO:0005635 (nuclear envelope), GO:0120114 (Sm-like protein family complex), GO:0070603 (SWI/SNF superfamilytype complex), GO:0090734 (site of DNA damage), GO:0031300 (intrinsic component of organelle membrane), GO:0032588 (trans-Golgi network membrane), GO:0042470 (melanosome), and GO:0001650 (fibrillar center) were significantly associated with the YTHDF alterations in LUAD patients (Figure 5). We also found out that YTHDF mutations predominantly affected the molecular functions, such as GO:0003713 (transcription coactivator activity), GO:1990247 (N6-methyladenosine-containing RNA binding), GO:0003682 (chromatin binding),GO:0032452 (histone demethylase activity), GO:0003725 (double-stranded RNA binding),GO:0043130 (ubiquitin binding), GO:0000978 (RNA polymerase II proximal promoter sequence-specific DNA binding), GO:0003729 (mRNA binding), GO:0004386 (helicase activity), GO:0015631 (tubulin binding), GO:0051219 (phosphoprotein binding), GO:0019904 (protein domain specific binding), GO:0042826 (histone deacetylase binding), GO:0003697 (singlestranded DNA binding), GO:0008168 (methyltransferase activity), GO:0003924 (GTPase activity) and GO:0004674 (protein serine/ threonine kinase activity) (Figure 5). In KEGG analysis, we found that 4 pathways were most frequently altered, including hsa03040 (Spliceosome), hsa05120 (Epithelial cell signaling in Helicobacter pylori infection), hsa05168 (Herpes simplex infection), and hsa04141(Protein processing in the endoplasmic reticulum) (Figure 5).
We studied and compared the expression, mutation, and prognostic values of different YTHDFs in two common non-small cell lung cancers: LUAD and LUSC. We found that the mRNA of the YTHDF family was overexpressed in lung tissues both from LUAD and LUSC patients. Our results showed that the lower mRNA expression of the YTHDF family was significantly associated with worse OS in overall lung cancer patients and LUAD patients, but not in LUSC patients, indicating that the YTHDFs mediated biological functions may have more critical roles in the progression of LUAD than in the LUSC. mRNA methylation is regulated by methyltransferases, RNA binding proteins, and demethylases [11,12]. The writer is composed of the enzymatic core components METTL3 and METTL14 and auxiliary proteins WTAP, VIRMA, FLACC, RBM15, and HAKAI. The writer is composed of YTHDF1/2/3, YTHDC1/2, and METTLs [11,12,25,26]. The binding of readers results in alterations of the translation efficiency and stability of m6A-containing RNAs [27]. FTO and ALKBH5 serve as eraser proteins that remove the methyl group [28].
Increasing studies on these regulators construct an increased association between m6A regulatory abnormalities or genetic alterations and various human cancers [7,8,29]. M6A demethylase FTO is reported as a prognostic factor in LUSC and facilitates cell proliferation and invasion despite inhibiting cell apoptosis by regulating MZF1 expressions [30]. METTL3 utilizes increased EGFR and TAZ expressions and promotes cell growth, survival, and invasion in order to facilitate a role as an oncogene in lung cancer [31]. mRNA circularization caused by METTL3-eIF3 promotes the translation and oncogenesis of LUAD [32]. SUMOylation of METTL3 is of significance for its promotion of tumor growth at lysine residues K177, K211, K212, and K215 in NSCLC [33]. m6A demethylase ALKBH5 inhibits tumor growth and metastasis by reducing YTHDFs-mediated YAP expression and inhibiting miR- 107/LATS2–mediated YAP activity in NSCLC [34]. Furthermore, m6A gives greater potential for early diagnosis and treatment of cancers. There are widespread genetic alterations in m6A regulators across 10,000+ subjects and 33 cancer types and regulator expression levels are significantly correlated with the activity of cancer hallmark-related pathways [35]. Thus, we focused on the expression and role of YTHDF1/2/3, an m6A reader family, in NSCLC. We compared the expression, mutation, and prognostic values of YTHDF1/2/3 in two common non-small cell lung cancers: LUAD and LUSC. Unlike other cancers, the overall average mutation frequency of YTHDF1/2/3 in LUAD patients was much higher, ranging from 19-36%. The significant prognostic values difference between LUAD and LUSC suggested that YTHDF1/2/3 are potential translational medicine targets for LUAD, but not for LUSC. By using the Kaplan-Meier Plotter, we also analyzed the predicted effect of YTHDFs in different subclasses and treatments of LUAD patients. However, the cohort numbers in patients from stage 3, and patients with chemotherapy were relatively small. Thus, the statistical analysis from these groups were not accurate. Further analysis by using larger cohorts would be necessary to address the prognostic role of YTHDFs in LUAD patients in these subclasses.
YTHDF1 is based in the cytoplasm, where the m6A binding protein facilitates the translation efficiency of m6A-modified mRNAs [36]. YTHDF2, after being targeted to a specific site via m6A recognition, recruits the CCR4-NOT deadenylase complex to destabilize and further decay target m6 A-modified transcripts [27]. YTHDF2 also serves m1A readers and destabilizes known m1A-containing RNAs [37]. YTHDF3 is another cytoplasmic m6A binder that promotes protein synthesis in synergy with YTHDF1 and affects methylated mRNA decay mediated through YTHDF2 [15]. This indicates that YTHDF3 plays critical roles in accelerating the metabolism of m6A-modified mRNAs in the cytoplasm while in conjunction with YTHDF1 and YTHDF2 proteins [15]. However, observances in m6A perturbations attributed to m6A regulators such as these implicate a close association between aberrant m6A modifications and human cancers. m6A reader YTHDF3 is reported to correlate with the activation of several oncogenic pathways including protein secretion, androgen response, and the TGF-β signaling pathway [35,38]. In this study, we proved that lower expressions of YTHDF1/2/3 predict worse survival conditions in LUAD patients, suggesting that YTHDFs suppressed the pathological progression in LUAD. YTHDF1/2 have been proved to inhibit the proliferation and migration of endosomal cancer cells via PHLPP2 and MTOR2 dependent pathways [7,39,40].
By using the protein network analysis, we identified that the expression of MTOR and other mRNA metabolic process genes were significantly related to YTHDFs mutations. This data suggests that the effect of YTHDFs on the suppression of LUAD may also occur through regulating MTOR dependent pathways. To address the detailed mechanisms of YTHDFs on LUAD progression, further molecular biological studies are still needed.
In summary, we systematically analyzed the expression profiles and prognostic values of the YTHDF1/2/3 in LUAD and LUSC patients. Our results revealed that YTHDF1, YTHDF2, and YTHDF3 might be useful markers for prognostic stratification for LUAD patients but not for LUSC patients. Our analysis also lays a foundation for the development of LUAD therapeutic strategies based on RNA methylation.
The authors have no conflicts of interest to declare.


