Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: August 30, 2022; Published: September 13, 2022
*Corresponding author: Gian Maria Pacifici, Associate Professor of Pharmacology, via Sant’Andrea 32, 56127 Pisa, Italy
DOI: 10.26717/BJSTR.2022.46.007305
The carbapenems used in paediatric patients are: imipenem with cilastatin, meropenem and ertapenem. Whereas imipenem and meropenem are extensively studied in infants and children little information is available for ertapenem. Imipenem is degraded by a renal tubular dipeptidase and imipenem is co-administered with cilastatin a drug that inhibits the degradation of imipenem. Imipenem binds to penicillin-binding protein, disrupts bacterial cell wall synthesis, causes death of susceptible microorganisms, and is very resistant to hydrolysis by most β-lactamases. Imipenem/cilastatin treats urinary-tract, respiratory-tract, intraabdominal, gynaecological, skin, soft-tissue, and joint infections. In preterm infants, the elimination half-life of imipenem and cilastatin is about 2.5 and 9 hours, respectively. Compared to imipenem, meropenem is somewhat less active against gram-positive organisms and more active against gram-negative organisms. The elimination half-life of meropenem ranges from 0.8 to 1.6 hours and is longer in infants than in older children. The treatment of bacterial infections and the treatment of bacterial meningitis with meropenem have been reported. Meropenem poorly crosses the human placenta and poorly migrates into the beast-milk. Ertapenem has a longer elimination half-life than imipenem and meropenem that allows once-daily dosing. The aim of this study is to review the dosing, efficacy and safety, pharmacokinetics, treatment of bacterial infections, treatment of bacterial meningitis, penetration into the cerebrospinal fluid, transfer across the human placenta, and migration into the breast-milk of imipenem/cilastatin and meropenem, and also the efficacy, safety, pharmacokinetics, and treatment with ertapenem have been reported in children.
Keywords: Breast Milk; Cerebrospinal-Fluid; Cilastatin; Dosing; Efficacy-Safety; Ertapenem; Imipenem; Meningitis; Meropenem; Pharmacokinetics; Placenta; Treatment
The carbapenems used in paediatric patients are: imipenem with cilastatin, meropenem, and ertapenem.
Mechanism of action, dosing, and use of carbapenems.
Imipenem is marked in combination with cilastatin, a drug that inhibits the degradation of imipenem by a renal tubular dipeptidase. Imipenem, like other β-lactam antibiotics, binds to penicillin-binding proteins, disrupts bacterial cell wall synthesis, causes death of susceptible microorganisms, and imipenem is very resistant to hydrolysis by most β-lactamases. The activity of imipenem is excellent in-vitro for a wide variety of aerobic and anaerobic microorganisms. Streptococci (including penicillinresistant Streptococcus pneumoniae), enterococci (excluding Enterococcus faecium and non-β-lactamase-producing penicillinresistant strains), staphylococci (including penicillinaseproducing strains but not methicillin-resistant Staphylococcus aureus), and Listeria (although ampicillin is more active) all are typically susceptible. Imipenem activity is excellent against the Enterobacteriaceae with the exception of emerging Klebsiella pneumoniae carbapenemase-producing strains. Most strains of Pseudomonas, Acinetobacter, and Anaerobes including Bacillus fragilis are highly susceptible. Imipenem also displays activity against Nocardia species and some species of rapidly growing mycobacteria. Imipenem is not absorbed orally. The drug is hydrolysed rapidly by a dipeptidase found in the brush border of the proximal tubule. When imipenem is administered concurrently with cilastatin, about 70% of administered imipenem dose is recovered in the urine as the active drug. Imipenem/cilastatin effectively treats a wide variety of infections, including urinarytract, respiratory-tract, intraabdominal, gynaecological, skin, tissue, bone, and joint infections. This drug combination appears to be especially useful for the treatment of infections caused by cephalosporin-resistant nosocomial bacteria. When imipenem is used for treatment of severe Pseudomonas aeruginosa infections, resistance may develop during therapy [1] (Figures 1 & 2).
Meropenem is a derivative of thienamycin. It does not require co-administration with cilastatin because it is not sensitive to renal dipeptidase. Compared to imipenem, meropenem is somewhat less active against gram-positive organisms (particularly Enterococcus) and more active against gram-negative organisms. Meropenem toxicity is similar to that of imipenem except that meropenem may be less likely to cause seizures; thus, it is preferred for treatment of bacterial meningitis when carbapenems therapy is required [1].
Ertapenem differs from imipenem and meropenem by having a longer elimination half-life that allows once-daily dosing and by having inferior activity against Enterococcus, Pseudomonas aeruginosa, and Acinetobacter species. Ertapenem activity against Enterobacteriaceae and anaerobes makes it useful treatment of intraabdominal and pelvic infections [1].
The literature search was performed electronically using PubMed database as search engines. The following key words were used: Imipenem children, meropenem children, and ertapenem children. In addition, the book “The Pharmacological Basis of the Therapeutics” [1] has been consulted.
Intravenous infusion of imipenem to treat aerobic and anaerobic gram-positive and gram-negative infections (not indicated for central nervous system infections), and hospitalacquired septicaemia.
• Newborns aged up to 7 days. Give: 20 mg/kg twice-daily.
• Newborns aged 7 to 20 days. Give: 20 mg/kg thrice-daily.
• Newborns aged 21 to 28 days. Give: 20 mg/kg 4 timesdaily.
Intravenous infusion of imipenem to treat aerobic and anaerobic gram-positive and gram-negative infections (not indicated for central nervous system infections), and hospitalacquired septicaemia
• Children aged 1 to 2 months. Give: 20 mg/kg 4 times-daily.
• Children aged 3 months to 17 years. Give: 25 mg/kg 4 times-daily.
Intravenous infusion of imipenem to treat infections caused by Pseudomonas, other sensitive organisms, infection in febrile children with neutropenia, and life-threatening infection
• Children aged 3 months to 17 years. Give: 25 mg/kg 4 times-daily (maximum per dose = 1 gram).
Intravenous infusion of imipenem to treat children with cystic fibrosis
• Children. Give: 25 mg/kg 4 times-daily (maximum per dose = 1 gram).
Imipenem/cilastatin was administered at a dose of 100 mg/ kg to infants and children, aged up to 3 years, and at a dose of 60 mg/kg to children aged more than 3 years. Imipenem/cilastatin effectively and safety treats infections caused by a broad spectrum organisms including Haemophilus influenzae, Staphylococcus aureus, or Pseudomonas aeruginosa [3]. Imipenem/cilastatin was intravenously infused to 25 infants and children, aged 5 months to 11 years, with acute osteomyelitis (N = 7), suppurative arthritis (N = 11) or both (N = 7). Imipenem/cilastatin infusion is effective and well-tolerated and treats these diseases. This drug combination effectively also treats acute bone and joint infections in paediatric patients [4].
Table 1: Pharmacokinetic parameters of imipenem which have been obtained in 39 preterm infants. Values are the mean+SD, by Reed, et al. [5].

Note: T1/2 = elimination half-life. DVss = distribution volume at steady-state. AUC = area under the concentration-time curve. TBC = total body clearance. X = amount excreted unchanged in the urine over 12 hours period. Clr = renal clearance. Clnr = non-renal clearance
Table 2: Pharmacokinetic parameters of cilastatin which have been obtained in 39 preterm infants. Values are the mean+SD, by Reed, et al. [5].
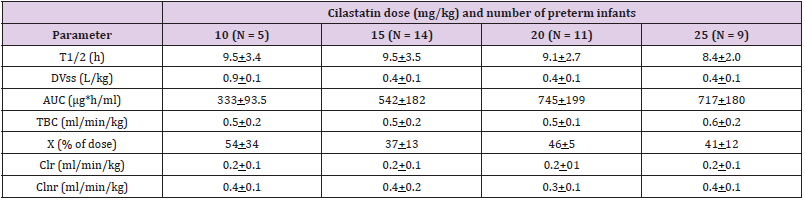
Note: T1/2 = elimination half-life. DVss = distribution volume at steady-state. AUC = area under the concentration-time curve. TBC = total body clearance. X = amount excreted unchanged in the urine over 12 hours period. Clr = renal clearance. Clnr = non-renal clearance
Table 3: Comparison of imipenem and cilastatin elimination between preterm infants and healthy adults. Values are the mean, by Reed, et al. [5].
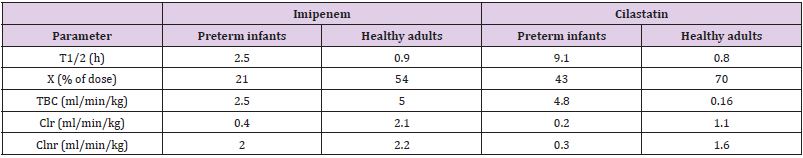
Note: T1/2 = elimination half-life. X = amount of drug excreted in the urine over the study interval. TBC = total boy clearance. Clr = renal clearance. Clnr = nor-renal clearance.
Reed, et al. [5] studied the pharmacokinetics of imipenem in 39 preterm infants with a gestational age, postnatal age, body-weight, surface area, and serum creatinine of 22 to 36 weeks (mean, 29.0+3.2), 1 to 6 days (mean, 2.6+1.1), 670 to 1,890 grams (mean, 1.187+386), 0.08 to 0.15 m2 (mean, 0.11+0.02), and 0.4 to 1.4 mg/ dl (mean, 1.1+0.2), respectively, and imipenem/cilastatin (1:1) was administered intravenously at a dose of 10, 15, 20, and 25 mg/kg (Table 1). This table shows that the area under the concentrationtime curve increases with the dose whereas the other parameters do not vary with the dose, the non-renal clearance is smaller than the total body clearance and the non-renal clearance, the distribution volume is lower than the water volume, and the amount of imipenem excreted with the urine over 12 hours is about one quart of the dose. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation of infant disease and age (Table 2). This table shows that the area under the concentration-time curve increases with the dose whereas the other parameters do not vary with the dose, the renal clearance is smaller than the total body clearance and non-renal clearance, the distribution volume is lower than the water volume, and the amount of imipenem excreted with the urine over 12 hours is about one half of the dose. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation of infant disease and age (Table 3).
This table shows that the elimination half-life of both imipenem and cilastatin is shorter in healthy adults than in preterm infants, the amounts of both imipenem and cilastatin excreted in the urine are higher in healthy adults than in preterm infants, the total body clearance of imipenem is higher in healthy adults than in preterm infants, that of cilastatin is lower in healthy adults than in preterm infants, the renal clearance of both imipenem and cilastatin is higher in healthy adults than in preterm infants, the non-renal clearance of imipenem is similar in healthy adults and in preterm infants whereas that of cilastatin is higher in heathy adults that in preterm infants. Imipenem and cilastatin are eliminated by renal elimination and the renal function is impaired in preterm infants thus the elimination parameters of imipenem and cilastatin are better expressed in healthy adults than in preterm infants.
Imipenem/cilastatin (1:1) was intravenously infused at a dose of 20 mg/kg to 25 preterm infants with septicaemia caused by Acinetobacter calcoaceticus (N = 7), Enterobacter cloacae (N = 5), methicillin-resistant Staphylococcus aureus (N = 5), other gram-negative bacilli (N = 5), Staphylococcus epidermidis (N = 2), and Klebsiella pneumoniae (N = 1) and the clinical cure was achieved in 17 of 25 infants (68%) and improvement was noted in an additional 3 infants (12%) while 5 infants (20%) were evaluated as treatment failures and no adverse-effects were noted thus imipenem/cilastatin successfully and safety treats bacterial infections in preterm infants [6]. Sixty-one hospitalized infants, aged 1 day to 6 months, had bacterial infections and were treated with imipenem/cilastatin intravenously at a dose of 50 mg/ kg twice-daily given for 11 days and this treatment successfully treats the infections in all infants [7]. Forty-five infants had an infection caused by Klebsiella pneumonia and were treated with imipenem/cilastatin intravenously and imipenem/cilastatin treats the infection in all infants [8]. Thirty-one infants and children with life-threatening hospital-acquired infections were treated with imipenem/cilastatin intravenously at a daily dose of 50 mg/kg. The therapy was well-tolerated and successfully treats the infections in all infants and children [9].
The penetration of imipenem into the CSF was evaluated in 20 children, aged 4 months to 11 years, with bacterial infections in the central nervous system. Ten children (50%) received a single imipenem intravenous dose of 25 mg/kg and 10 children (50%) received three imipenem doses of 25 mg/kg intravenously 4 times-daily. Imipenem concentrations after single-dose infusion in serum and CSF during the early phase of treatment were 8.59+0.95 and 1.36+0.32 μg/ml, respectively, were similar to those during the late phase (9.96+2.36 and 2.08+1.14 μg/ml, respectively). Concentrations of imipenem in serum and CSF after multiple-dose infusion during the early phase were 11.97+2.03 and 1.87+0.29 μg/ml, respectively, were similar to those during the late phase (9.57+1.76 and 1.22+0.11 μg/ml, respectively). A mean CSF penetration-rate of imipenem was 15 to 27%. These findings suggest that imipenem sufficiently penetrates into CSF of children [10].
Fifty newborns and infants had the meningitis caused by Citrobacter diversus and imipenem/cilastatin successfully treats the meningitis caused by Citrobacter diversus in all newborns and infants [11]. Twenty-one newborns and children, age 3 to 48 moths, had the meningitis caused by Haemophilus influenzae type b and imipenem/cilastatin successfully treats the meningitis in all newborns and children [12].
Intravenous infusion or intravenous injection of meropenem to teat infections due to aerobic and anaerobic gram-positive and gram-negative bacteria or hospital-acquired pneumonia.
• Newborns aged up to 7 days. Give: 20 mg/kg twice-daily.
• Newborns aged 7 to 28 days. Give: 20 mg/kg thrice-daily.
Intravenous infusion or intravenous injection of meropenem to teat infections due to aerobic and anaerobic gram-positive and gram-negative bacteria or hospital-acquired pneumonia
• Children aged 1 moth to 11 years, with body-weight up to 50 kg. Give: 10 to 20 mg/kg thrice-daily.
• Children aged 1 month to 11 years, with body-weight of 50 kg and above. Give: 0.5 to 1 gram thrice-daily.
• Children aged 12 to 17 years. Give: 0.5 to 1 gram thricedaily.
Intravenous infusion or intravenous injection of meropenem to treat severe infections due to aerobic and anaerobic gram-positive and gram-negative bacteria
• Newborns aged up to 7 days. Give: 40 mg/kg twice-daily.
• Newborns aged 7 to 28 days. Give: 40 mg/kg thrice-daily.
Intravenous infusion or intravenous injection of meropenem to treat exacerbations of chronic lower respiratory-tract in cystic fibrosis children
• Children aged 1 month to 11 years, with body-weight up to 50 kg. Give: 40 mg/kg thrice-daily.
• Children aged 1 month to 11 years with body-weight of 50 kg and above. Give: 2 grams thrice-daily.
• Children aged 12 to 17 years. Give: 2 grams thrice-daily.
Intravenous infusion of meropenem to treat bacterial meningitis
• Newborns aged up to 7 days. Give: 40 mg/kg twice-daily.
• Newborns aged 7 to 28 days. Give: 40 mg/kg thrice-daily. Intravenous infusion of meropenem to treat bacterial meningitis
• Children aged 1 month to 11 years, with body-weight up to 50 kg. Give: 40 mg/kg thrice-daily
• Children aged 1 month to 11 years, with body-weight of 50 mg and above. Give: 2 grams thrice-daily.
• Children aged 12 to 17 year. Give: 2 grams thrice-daily.
Meropenem was well-tolerated in 200 critically ill infants, aged less than 90 days, the effectiveness of treatment was evaluable in 96% of infants and overall treatment success was observed in 84% of infants [16]. Infants and children, aged 2 months to 12 years, with symptoms of respiratory-tract infection, urinary-tract infection, septicaemia, skin, and intraabdominal infections were enrolled. Meropenem was well-tolerated and effectively treats these infections in all infants and children [17].
Meropenem was well-tolerated in 200 critically ill infants, aged less than 90 days, the effectiveness of treatment was evaluable in 96% of infants and overall treatment success was observed in 84% of infants [16]. Infants and children, aged 2 months to 12 years, with symptoms of respiratory-tract infection, urinary-tract infection, septicaemia, skin, and intraabdominal infections were enrolled. Meropenem was well-tolerated and effectively treats these infections in all infants and children [17].Blumer, et al. [18] studied the pharmacokinetic of meropenem in 63 infants and children (38 males and 25 females). The patients were aged from 0.25 to 12.3 years (mean, 4.0+3.5), the patient body-weight ranged from 3.7 to 45 kg (mean, 16.5+11.0), the serum creatinine concentration ranged from 0.2 to 1.1 mg/dl (mean, 0.45+0.2), and the total bilirubin concentration ranged from 0.0 to 2.4 mg/dl (mean, 0.46+0.5). Patients were stratified into four groups by age: 2 to 5 months, 6 to 23 months, 2 to 5 years, and 6 to 12 years and a single intravenous administration of meropenem of 10, 20, or 40 mg/kg was administered (Table 4). This table shows that meropenem is rapidly eliminated as the mean elimination half-life is about 1 hour, the distribution volume is lower than the water volume, the renal clearance is about one half of the total body clearance and the bioavailability is about 0.5 but this parameter reduces with the dose increasing. The other pharmacokinetic parameters are not dose-dependent. In addition, there is a remarkable interindividual variability of the pharmacokinetic parameters and this variability is accounted by a wide variation in patient disease and age (Table 5). This table shows that mean elimination half-file and the mean residence time of meropenem reduce with the increasing of subject age. Meropenem is eliminated by renal route and the renal function increases with infant maturation and child development thus the elimination half-life decreases with the increasing of subject age. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by the wide variation in patient disease and age (Table 6).
Table 4: Comparison of imipenem and cilastatin elimination between preterm infants and healthy adults. Values are the mean, by Reed, et al. [5].
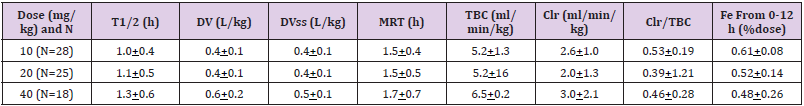
Note: T1/2 = elimination half-life. DV = distribution volume. DVss = distribution volume at steady-state. MRT = mean residence time. TBC = total body clearance. Clr = renal clearance. Fe = bioavailability from 0 to 12 hours.
Table 5: Pharmacokinetic parameters of meropenem which have been obtained in 63 infants and children. Effect of the age on the pharmacokinetic parameters. Values are the mean+SD, by Blumer, et al. [18].
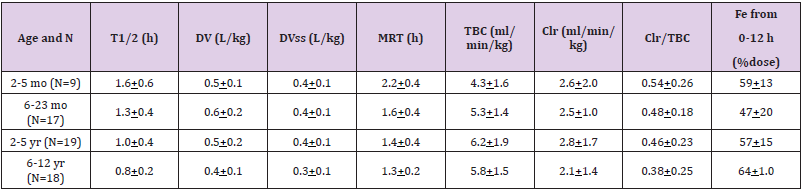
Note: T1/2 = elimination half-life. DV = distribution volume. DVss = distribution volume at steady-state. MRT = mean residence time. TBC = total body clearance. Clr = renal clearance. Fe = bioavailability from 0 to 12 hours.
Table 6: Pharmacokinetic parameter of ertapenem which have been obtained in 41 infants, 28 children and 11 adolescents. Values are the mean+SD, by Karaaslan, et al. [30].
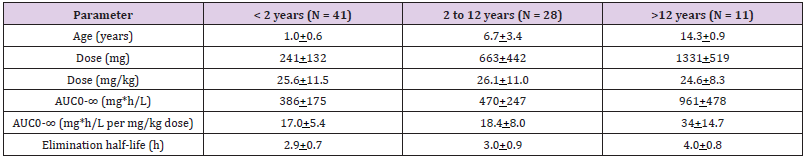
Note: T1/2 = elimination half-life. DV = distribution volume. DVss = distribution volume at steady-state. MRT = mean residence time. TBC = total body clearance. Clr = renal clearance. Fe = bioavailability from 0 to 12 hours.
Note: AUC = area under the concentration-time curve.
Meropenem effectively treats serious infections caused by multiply-resistant pathogens in infants and children [19]. Meropenem administered at doses of 120 and 60 mg/kg daily successfully treats bacterial infections in infants and children [20]. Meropenem is an effective and safe treatment of infants and children with serious urinary-tract, pneumonia, sepsis, intraabdominal, skin, and soft-tissue infections [21].
Meropenem penetrates into the CSF of 167 newborns and children in significant amounts and successfully treats bacterial meningitis in all newborns and children [22].
Seventy-nine infants and children received meropenem and 75 infants and children received cefotaxime. At the end of treatment there were no significant differences in clinical outcomes between the two treatment groups. Meropenem is a suitable therapy for bacterial meningitis in infants and children and has an efficacy and safety profile similar to that of cefotaxime [23].
The transfer of meropenem across the human placenta was studied using the placenta perfusion. Maternal and foetal mean peak concentrations of meropenem were 54.3+3.3 μg/ml and 2.2+0.18 μg/ml, respectively, and maternal and foetal mean trough concentrations were 12.7+1.3 μg/ml and 0.41+0.10 μg/ml, respectively. Transplacental passage of meropenem is thus incomplete [24].
A lactating woman received meropenem intravenously at a dose of 1 gram at 3 days postpartum. The highest and lowest meropenem concentrations were 644 ng/ml and 246 ng/ml, respectively (Figure 4). These results indicate that meropenem poorly migrates into the beast-milk [25].
Intravenous infusion of ertapenem to treat intraabdominal, acute gynaecological infections, and community-acquired pneumonia.
• Children aged 3 months to 12 years. Give: 15 mg/kg twicedaily (maximum per day = 1 gram once-daily).
• Children aged 13 to 17 years. Give: 1 gram once-daily.
Intravenous infusion of ertapenem to treat diabetic foot infections, skin, and soft-tissue infections.
• Children aged 13 to 17 years. Give 1 gram once-daily.
Ertapenem is highly effective for treating urinary-tract infections caused by extended-spectrum-β-lactamase-producing organisms in children [27]. Ertapenem successfully and safety treats complicated urinary-tract infections and community-acquired pneumonia infections in children [28]. One gram of ertapenem, given intravenously to children, is generally well-tolerated and has safety and tolerability profiles similar to those of piperacillintazobactam and ceftriaxone [29].
Abdel-Rahman, et al. [30] studied the pharmacokinetics of ertapenem in 80 infants and children, aged 3 months to 16 years and weighing 6.1 to 82.6 kg (mean, 31.3+22.7), and a single intravenous dose of ertapenem was administered. Table 6 shows that the elimination half-life of ertapenem is longer in adolescents than in infants, the area under the concentration-time curve increases with the dose and with subject age, and ertapenem is slowly eliminated as the elimination half-life ranges from 2.9 to 4.0 hours and is longer in adolescents than in infants.
Fifty children aged 38.6+36.9 months (range, 6 to 156) had the urinary-tract infection caused by extended-spectrum β-lactamaseproducing bacteria and were treated with ertapenem. Ertapenem successfully treats the infection in these children [31]. The penetration-rate of ertapenem is 7.1% and 2.4% in the inflamed and non-inflamed meninges, respectively. Ertapenem has excellent antibacterial activity in treatment of bacterial meningitis due to penicillin-sensitive or penicillin-resistant pneumococci [32].
The carbapenems used in paediatric patients are: imipenem with cilastatin, meropenem, and ertapenem. Whereas imipenem/ cilastatin and meropenem have been extensively studied in infants and children little information is available for ertapenem. Imipenem binds to penicillin-binding protein, disrupts bacterial cell wall synthesis, causes death of susceptible microorganism, and is very resistant to hydrolysis by most β-lactamases. The activity of imipenem is excellent in-vitro for a wide variety of aerobic and anaerobic microorganisms. Imipenem is active against streptococci (including penicillin-resistant Streptococcus pneumoniae), enterococci (excluding Enterococcus faecium and non-β- lactamase-producing penicillin-resistant strains), staphylococci (including penicillin-producing strains but not methicillinresistant Staphylococcus aureus), Listeria, Enterobacteriaceae (except for Klebsiella pneumoniae carbapenemase-producing strains), most strains of Pseudomonas Acinetobacter, anaerobes (including Bacillus fragilis), and Nocardia species. Imipenem is degraded rapidly by a dipeptidase found in the brush border of the proximal tubule and is co-administered with cilastatin an inhibitor of the dehydropeptidase. Imipenem/cilastatin successfully treats urinary-tract, respiratory-tract, intraabdominal, gynaecological, skin, soft-tissue, bone, and joint infections. Compared to imipenem, meropenem is less active against gram-positive organisms and more active against gram-negative organisms.
Ertapenem has a longer elimination half-life than imipenem and meropenem that allows once-daily dosing. Ertapenem is less active than imipenem/cilastatin and meropenem against Enterococcus, Pseudomonal aeruginosa, and Acinetobacter species. Ertapenem is active against Enterobacteriaceae and anaerobes and is a useful treatment of intraabdominal and pelvic infections [1]. The efficacy and safety of imipenem/cilastatin have been reported in infants and children [3,4] and the elimination half-life of imipenem and cilastatin is about 2.5 and 9 hours, respectively, in preterm infants [5]. Imipenem/cilastatin effectively treats bacterial infections [6- 9], imipenem penetrates into the cerebrospinal fluid in significant amounts [10], and imipenem/cilastatin treats bacterial meningitis [11-12]. Imipenem and cilastatin promptly cross the human placenta [13,14]. Meropenem has been found efficacy and safe in infants and children [16,17] and the elimination half-life of meropenem ranges from 0.8 to 1.6 hours and is longer in infants than in children [18]. Meropenem treats bacterial infections [20,21], penetrates into the cerebrospinal fluid in significant amounts [22], and treats bacterial meningitis [23]. Meropenem poorly crosses the human placenta [24] and poorly migrates into the breast-milk [25].
Ertapenem effectively and safety treats urinary-tract infections caused by extended-spectrum β-lactamase-producing bacteria [27], community-acquired pneumonia [28], and ertapenem is welltolerated and safe in children and the tolerability profile is similar to that of piperacillin/tazobactam [29] and ertapenem treats infection caused by extended-spectrum β-lactamase producing bacteria [31]. The penetration-rate of ertapenem is 7.1% and 2.4% in inflamed and non-inflamed meninges, respectively, and ertapenem treats bacterial meningitis due to penicillin-sensitiveresistant pneumococci [32]. The elimination half-life of ertapenem ranges from 2.9 to 4.0 hours and is longer in adolescents than in infants [30]. In conclusion, the carbapenems used in paediatric patients are: imipenem with cilastatin, meropenem, and ertapenem. Whereas imipenem/cilastatin and meropenem have been extensively studied in infants and children little information is available for ertapenem. The elimination half-life of imipenem and cilastatin is about 2.5 and 9 hours, respectively, in preterm infants. Imipenem/cilastatin has been found efficacy and safe in infants and children, treats bacterial infections, imipenem penetrates into the cerebrospinal fluid in significant amounts, and imipenem/cilastatin treats bacterial meningitis. Imipenem and cilastatin promptly cross the human placenta. The elimination half-life of meropenem ranges from 0.8 to 1.6 hours and is longer in infants than in children.
Meropenem has been found efficacy and safe in infants and children, treats bacterial infections, penetrates into the cerebrospinal fluid in significant amounts, treats bacterial meningitis, is poorly transferred across the human placenta, and poorly migrates into the breast-milk. The elimination half-life of ertapenem ranges between 2.9 to 4.0 hours and is longer in adolescents that in infants, ertapenem has been found efficacy and safe in children, and ertapenem treats bacterial infections and bacterial meningitis in children. The aim of this study is to review the clinical pharmacology of carbapenems in infants and children.
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


