Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ephrem Shimelis*
Received: September 04, 2022; Published: September 15, 2022
*Corresponding author: Ephrem Shimelis, Addis Ababa University, College of Veterinary Medicine and Agriculture, Department of Veterinary Microbiology, Immunology and Veterinary Public Health, Ethiopia
DOI: 10.26717/BJSTR.2022.46.007314
The high prevalence of multidrug–resistant E. coli in table chicken egg shell supplied to the market center and shop from which the society can purchased for their food consumption, reflects a reservoir of resistance and easily transmission to humans. the objective of the current study was determining the prevalence and antimicrobial resistant status of E. coli in commercial chicken eggs in Bishoftu town, east, Shewa, Ethiopia. Between June 2021 to September 2021. Egg shells swabs egg internal contents were sampled purposively from the Bishoftu town market center and local shops. The E. coli was isolated by conventional bacteriological culturing on selective media and biochemical tests. The colony characterized as E. coli was subjected to antimicrobial susceptibility tests. From 92 samples, the E. coli was recovered from eleven (11.95%) of the egg samples collected from the marketing centers and shop in Bishoftu town, East Shewa, Ethiopia. out of 40-egg shell swab collected from the market center two (5%) of them are contaminated with e coli while out of 52 egg shell swabs collected from the shop 9 (17.3%) was contaminated by E coli with the significant differences between the marketing center and shops (P-Value = 0.009). the prevalence Escherichia coli in the Shell eggs and egg contents were (11.9 and 5.2%, respectively) with the significant statistical difference (P-Value = 0.0002).
All isolates (100%) were multi-drug resistance as eight (72.7%) of isolates were resistant to five different antimicrobials used and three (27.3%) of isolates were resistance the eight different antimicrobials used. The prevalence of antimicrobial resistance among the tested antibiotics was significantly different (p < 0.02). Improvement in the hygienic conditions of poultry farms and control of the misuse and overuse of antibiotics in poultry is therefore strongly recommended.
Keywords: Escherichia Coli; Eggs; Antibiotics Sensitivity
Poultry production is one of the economically important agricultural sectors used to generate eggs and meat to alleviate protein scarcity in Ethiopia [1]. According to the Moln [2] in 2918 as the demand for animal protein was increased the Global hen egg production was increased by 24.4% and chicken egg production is estimated to be 76.8 million tons per a year, despite the sector is challenged by the development and the emergence of public health important multidrug resistant bacteria in the poultry production chain [3]. Consumption of raw or unsafe food is contributing factors to an outbreak of foodborne disease in human [4]. Up to 2050, 10 million deaths per year will Globally, unless immediate action to prevent antimicrobial resistance (AMR) will be taken [5]. Escherichia coli O157:H7 (E. coli O157:H7) is a very important food-borne pathogen to human, especially from animal products [6]. All species of E. coli isolates are not cause the disease to the animals and harmless; only some strains are pathogenic and may cause serious food poisoning [7]. Escherichia coli is an important opportunistic pathogen that persist as a commensal microorganism in healthy humans and animals [8].
The pathogenic strains of E. coli can cause serious illness of urinary tract infections, bloodstream infections, skin infection, otitis media and diarrhea with antimicrobial resistance to antimicrobial for treatment of infected individual [9]. A freshly laid hen’s egg is devoid of microorganism, but soon after oviposition, it is contaminated by various spoilage and pathogenic microorganisms [10]. Unless all the necessary precautions are taken along the poultry production, marketing and processing chains, poultry meat and eggs can be contaminated by infectious agents that are harmful to humans [11]. Most retailers do not store eggs in refrigerators; thus, the eggs are exposed to weather conditions, resulting in contamination [11,12]. Table eggs are the primary source of protein in human diet that may be a cheap and easily available in both urban and rural area, despite it contaminated with pathogenic E. coli species that showed multiple drug resistance to antimicrobial agents commonly used in veterinary and human practice [7-13], In an effort to minimize these various types of contamination, regulations have been established to control the egg industry. However, in some regions of the world enforcement is lax and compliance is poor [14].
The egg shells and egg contents can be contaminated by the bacteria, either horizontal or vertical transmission [15,16]. Antimicrobial resistance through the food of animal origin contaminated with drug resistant microorganism is an important One Health challenge that encompasses the human, animal, and environmental fields [8-17]. The challenge of antimicrobial drug resistance Animal and human is a global threat for treatment of infectious diseases and costs life and money and threatens health delivery system’s effectiveness [9]. Antibiotics used as to increase feed conversion ratio, to promote growth of birds, to prevent, treat and control diseases and to combats stress from environmental changes in poultry farms, but he frequents exposure of pathogenic bacteria unrecommended dose of this antibiotics led to the emergence of antibiotics resistance [18]. These pathogenic microorganisms resistant to antimicrobial drugs may invade eggs and transmitted to human during their consumption [14]. The highest prevalence of resistance was observed for the groups of antimicrobials more frequently used on poultry farms and there is highest risk of transmission to humans through contaminated eggs [19,20]. Currently, antibiotic-resistant through contaminated eggs are become the public health concern from food safety point of view [19]. many communities in Ethiopia have a culture of drinking raw egg as a medication for respiratory and other illnesses [10]. Also, in Ethiopia, the table eggs are sold in marketing center and shops within the area it was overcrowded with other commodity, too many customers hand contact and environmental condition harbor pathogenic microorganisms including antimicrobial resistant E. coli. In Ethiopia, huge microbial contamination of eggs from retailers at urban settings could be due to unhygienic handling of the eggs, lack of standard storage and transportation facilities [21]. Only a few studies were conducted on the prevalence of e coli on the eggs provided for community at marketing centers and shops for consumers in Ethiopia, despite the prevalence of e coli from market egg was 45/83 (54.23%) with the incidence of E. coli in egg shell and egg content was 10% (6 of 60) and 1.67% (1 of 60), respectively in Ethiopia [10-22]. Antimicrobial resistance of E. coli in developing countries including Ethiopia is reported to be one major reason for failure of treatment of infectious diseases [9]. In an effort to minimize these various types of contamination, regulations have been established to control the egg industry. However, in some regions of the world enforcement is lax and compliance is poor set rules and provide routine monitoring for testing and controlling the unnecessary and unconscious use of antibiotics in the diet of chicken breeding [23]. Therefore, this study was aimed to determine the prevalence and antimicrobial resistance of E. Coli isolated from the Egg collected from Bishoftu Market Centers and shop
This cross-sectional study was conducted between May 2021 and June 2022 by collecting the egg shell swab and egg internal contents collected from open marketing center and shops in Bishoftu town, east Shewa, located in central Ethiopia at 47 km Southeast of Addis Ababa at an altitude of about 1900 nast (38° 58′′ E 08° 44′′ N) harboring a number of commercial and small-scale poultry farms. It has an annual rainfall of 1115.6 mm (NMSA, 2003). The marketing center and shop selected for the study were the area on which the community is exchange the commodity including the hens and hen eggs provided to them from the rural areas and intensive farms in Bishoftu town. The marketing center was overcrowded community, animals and plant dry and liquid product exchanged on fields with low drainage managements, dirty material and too much customer (buyer) hand contact before the buyer and seller negotiation. Also, the egg was provided to the consumer in Bishoftu through the shop in which the eggs can stored and sold with other shop commodity.
The correctional study design on the conventional isolation, identification, and antimicrobial sensitivity test of E. coli from the shells of egg supplied to the market center and shops in Bishoftu town from June 2021 up to September 2021. Randomly 52 eggs shell swab and 40 of the egg were collected from Bishoftu town markets center and shops. the eggs with visible fecal shell contamination were not taken as samples. The collected eggs shell swab with peptone water were transported to laboratory under cold chain and incubated for 24 hr under 37oC. Then, the samples were isolated by culturing in an eosin methylene blue agar (EMBA) medium. The indicator of E. coli bacteria is the round-shape of colony with a color of metallic green and black color in the center. The isolation was continued by performing Gram staining to confirm the E. coli bacteria. Then, the confirmation was followed by indol, methyl red, voges proskauer and citrate (IMVIC) test. For further test, the confirmed bacteria of E. coli were cultured in selective medium sorbitol MacConkey agar (SMAC) and incubated for 18h-24h. It was aimed to identify E. coli, which is indicated by clear appearance of colony and colorless or sorbitol negative. Finally, antimicrobial sensitivity to six available drug disks were conducted.
Isolation and Identification of E Coli: A loop from incubated peptone water of egg shells were streaked on EMB agar and incubated for 24 hr at 37°C. Then the plates were examined for the presence of colonies that may resemble E. coli according to the technique recommended by Germini et al. 2009. The organisms showing characteristic colony morphology of E. coli on EMB agar having black golden on the center of colonies were isolated. Isolated colonies were taken directly from the plate and transferred to Sorbitol MacConkey to notice the typical E. coli O157:H7 colonies after overnight culture (24hrs) at 37˚C. E. coli isolates that appeared pink on Rainbow O157 and non-sorbitol fermenting on CT-SMAC were presumed as presumptively E. coli O157:H7 colonies.
Biochemical Characterization of E Coli: The isolated E. coli were then subjected towards biochemically tested using the IMVIC (indole, methyl red, Voges Proskauer, and citrate) test and on triple sugar iron agar (TSI) and motility. The E. coli isolates revealed a complete fermentation of 5 basic sugars by producing both acid and gas. The isolates were also revealed a positive reaction to the MR test and the Indole test but a negative reaction to the VP test. TSI agar medium gave yellow color in both the slant and butt with gas production.
Antimicrobial Sensitivity Test: Identified E. coli with biochemical test were taken for antimicrobial patters with sub culturing on nutrient agar medium. Seven [24] antimicrobial discs of clinically utilizing drug were applied for each isolate with qualitative agar diffusion method (Kirby-Bauer method) by employing Mueller Hinton agar medium. Culture of each isolated were compared with 0.5 McFarland turbidity standards. Isolates were swabbed on Mueller Hinton agar using sterile swabs. Antimicrobial impregnated discs were seated on the surface of cultures of Muller-Hinton agar and incubate at 37°C for 20 h intended for E. coli isolates taste for susceptibility to the antimicrobial using the disk diffusion method according to guidelines set by the clinical laboratory standards institute (CLSI). For each antimicrobial, mm of inhibition zone was measured by using digital vernier calipers and inhibition zone of each antimicrobial were classified (resistant, intermediate, or susceptible).
Data Management and Analysis: The laboratory analysis was entered into the Microsoft-Excel spreadsheet. Descriptive statistics were used to summarize the data in excel. The association between the prevalence of E. coli infection in dogs and potential risk factors was first analyzed using a one-way anova analyses. A likelihood ratio test with a P-value of less than 0.05 suggested an interaction between the two variables being tested and was retained in the final model. In all the analyses P-value of less than 0.05 was considered significant.
Out of 92 samples collected from the marketing centers and shop in Bishoftu town, the E. coli was recovered from eleven (11.95%) of a sample. The prevalence of the E. coli in the marketing center and shops were 5% and 17.3% respectively with the significant differences between the marketing center and shops (P-Value = 0.009) (Table 1). The prevalence of E. coli in the egg shells swab and egg yolks examined for the isolation of E. coli indicated that there was high prevalence in egg shells as the prevalence in the egg shells and egg yolk were 13.4% and 0%, respectively with the statistical difference (P-Value = 0.0002) (Table 2).
Table 1: Interpretation of zones of inhibition (in mm) for Kirby-Bauer antibiotic susceptibility test.
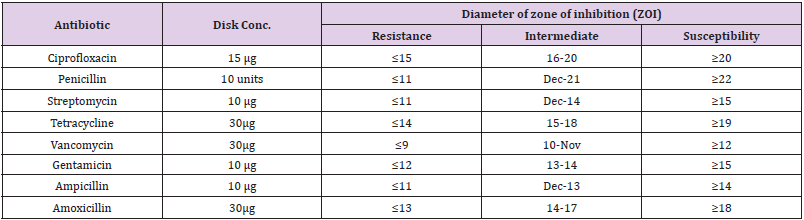
A total of 11 of E. coli isolates were subjected to the eight [11] antimicrobials following CLSI guidelines. Out of 11 E. coli isolates, all isolates (100%) were susceptible to ciprofloxacin, 90.9% susceptibility to Gentamycin and 81.6% to the streptomycin, however, the isolates were resistance to the Tetracycline, penicillin, Amoxicillin, Ampicillin, and vancomycin. (Table 3). There was significant resistance of the prevalence of antimicrobial resistance of E. coli isolated from the egg shell to the eight antibiotics used in the current study was significantly different (p < 0.0004) as the. Chi-squared test (X-squared = 26.138, df = 7) for eight antibiotics used. While the there was no statistical resistance difference of each (mono) to the eight-antimicrobial used in the study (p >0.05). All isolates (100%) were multi-drug resistance as eight (72.7%) of isolates were resistant to five different antimicrobials used and three (27.3%) of isolates were resistance the eight different antimicrobials used (Table 4). The prevalence of antimicrobial resistance among the tested antibiotics was significantly different (p < 0.02).
The development and transmission of antimicrobial resistance factors of the foodborne E. coli from the animals to the human through consumption of food of the animal origin is one of major public health concern across the worldwide [25,26]. One of the foods of animal origin that can play an important role on the transmission of these microorganism is the contaminated eggs during the egg laying process in contact with the pathogen in the intestine and reproductive tract of the infected birds and also be contaminated in contact with environmental bacteria after it laid [18]. According to the Okorie-kanu, et al. [27], the raw table eggs provided to the markets for human consumption can contaminated with multiple drug resistance E. coli to antimicrobial agents commonly used in veterinary and human practice. The eggs supply to the retailers at urban can exposes to the microorganism contamination due to unsafe handling of the eggs, lack of proper storage and transportation facilities [21]. This can expose the people to infected with multi-drug resistant zoonotic pathogenic bacterial including E. coli O157:H7. Therefore, the objective of the current study was determining the prevalence and antimicrobial resistant status of E. coli in commercial chicken eggs in Bishoftu town, east, Shewa, Ethiopia.
According to the objective, the conventional bacteriological E. coli isolation and antimicrobial susceptibility tests to determine the antimicrobial resistance was conducted on the egg shell swab and egg internal content collected from community marketing center and shops in Bishoftu town. From 92 samples, the E. coli was recovered from eleven (11.95%) of the egg samples collected from the marketing centers and shop in Bishoftu town, East Shewa, Ethiopia. This finding was almost in agreement with the report of Sarba, et al. [25] who reported the prevalence of 11.5% (80/694) [95% CI: 9.64–14.61] E.coli isolation rate at chicken organ level from backyard chicken in and around ambo, Central Ethiopia, but the result in the current study was lower than the prevalence of Escherichia coli (18% ) from the table eggs collected from Mansoura, Egypt reported by Elafify et al. (8) and higher than that reported by Atoyebi, et al. [14] who got the prevalence the total prevalence of E. coli O157:H7 was 9.8 %), with the All the E. coli O157:H7 isolated were from the egg shell. Also, the overall prevalence of our current study was relatively agreed with the prevalence of the E. coli O157:H7. 13.4% reported by [26] of cloacae swab samples from Poultry Farms, Eastern Ethiopia. The greater difference in prevalence of E.coli between our current study and that reported by Rind, et al. [7] who demonstrated an overall 37.00% E. coli contamination in table eggs.
The prevalence of E. coli was different between the sample types (egg shell swab and egg internal content) and sample origin (marketing center and shops). Accordingly, out of 40-egg shell swab collected from the market center two (5%) of them are contaminated with e coli while out of 52 egg shell swabs collected from the shop 9 (17.3%) was contaminated by E coli. The current result was agreed with the result obtained from brown shell egg with prevalence of egg contents (0%) and Baladi egg shell (12%). Reported by Elafify et al. (8) from table eggs in Mansoura, Egypt. The significant differences between the marketing center and shop (P-value < 0.05) on Table 1 could agree with the postulates’ of Fahim, [28] that revealed the critical role of storage temperature on egg quality parameters, as well as the great influence of the housing environment on the microbial profile of produced eggs. The contamination statistical difference between the sample collected between the sample source indicated that there is a significant between the market center and shops (P-Value < 0.05). Escherichia coli is not only regarded as an indicator of fecal contamination but more likely as an indicator of poor hygiene and sanitary practices [29]. The prevalence of E. coli in the egg shells swab and egg yolks examined for the isolation of E. coli indicated that there was high prevalence in egg shells as the prevalence in the egg shells and egg yolk were 13.4% and 0%, respectively with the statistical difference (P-Value = 0.0002) (Table 2). There was difference in the prevalence of this finding and report of Nahashon, et al. [30] that indicates the prevalence Escherichia coli in the Shell eggs and egg contents were (11.9 and 5.2%, respectively). This report is also different from the findings of Atoyebi, et al. [18] who reported lower prevalence of 9.8 % which was entirely from egg shell. Despite there was similarity in the egg contents was devoid of E. coli O157:H7. The prevalence of E.coli in egg internal content was agreed with the report of Kapena, [31] who revealed an overall contamination rate of 0% of E. coli from the internal egg contents.
The significant difference between egg shell and egg contents indicated that there was lower prevalence of E.coli internal content of the chicken egg was agreed with the experimental report of [32] showed that chicken egg cuticle is a main barrier against bacterial penetration in precocial birds’ eggs. For each individual bacterial strain, the mean cuticle deposition was lower for penetrated compared to non-penetrated eggshells [33]. The variation may be accounted for by the difference in the sample used. Whereas passage of egg through the chicken cloacae is expected to contribute to the contamination of egg shell. Most of bacterial contamination of eggs contents result from bacterial contamination of egg shells that invade egg contents under improper conditions [11]. The absence of the E. coli O157:H7 in egg content observed in this study may be as a result of the natural protective mechanism which makes contamination of egg content difficult. The egg shell and the outer shell membrane that separate the shell from the albumen serves as a major barrier to bacteria. In addition, the albumen gives a basic environment which discourages the proliferation of many bacteria [18].
The find¬ing of E. coli only on the shell, and none inside the egg, strongly intimates that E. coli contaminates the egg superficially, mainly through the fecal route by droppings, or during the hatching process through the cloacae. Further, E. coli may even contaminate the eggshell once it’s hatched in the outside environ¬ment of the chicken [31]. E. coli isolates were completely (100%) susceptible to ciprofloxacin, gentamicin (90.09%) and streptomycin (81.8%) as well as the100% resistance to amoxicillin the were relatively agreed with the report of Sarba, et al. [25] that E. coli isolates were completely (100%) susceptible to ciprofloxacin, to gentamicin (93%) and streptomycin (85%) and 100% resistance to the amoxicillin, however, 100% resistance to the tetracycline in the current finding was difference from resistance (46.3%) level of tetracycline of Sarba, et al. [25]. All of the E. coli isolates were susceptible to the Ciprofloxacin and gentamycin (GM). However, the 90.09% of isolates were susceptible in this study, according to the report of [8] the highest resistance rates of the Escherichia coli Isolates from Retail Poultry Products in Spain were detected for gentamicin (79%).this difference in resistance pattern due to the gentamycin uses as feed supplement and treatments between different countries. All E. coli O157: H7 isolates were 100% resistance to Tetracycline, penicillin, Amoxicillin, Ampicillin and vancomycin that can also be attributed to the uncontrolled/ widespread use of these antimicrobials as mainly growth promoters since the farmers have unlimited access to these agents and their use [27]. 100% resistance to penicillin tetracycline, rifampicin, strep¬tomycin, erythromycin, amoxicillin and vancomycin were agreed with Okorie-kanu, et al. [27] who observed in all iso¬lates of pathogenic E. coli and Salmonella spp. tested in this study. Both micro-organisms showed 100% resistance to penicillin. Also, this result was consistent with the report of Haque, et al. [34] in which all E. coli were found highly resistant to penicillin (100%), tetracycline 80.95%) and ampicillin (100%) and ciprofloxacin (85.71%), and gentamicin (95.23%). Multidrug resistance to more than two antimicrobial agents was detected in 11(100%) of the isolates in which the E. coli O157:H7 isolates, 72.7%, and 27.3% expressed resistance to five, and six antimicrobials, respectively (Table 4) agreements with the report of Garc, et al. [35] which indicated the 100% of the E. coli from Retail Poultry Products in Spain showed resistance to at least one antibiotic and relative agreement with multidrug resistance to more than two antimicrobial agents detected in 24 (92.30%) of the isolates reported by Shecho, et al. [26] however, it was a great different from the result reported by (13) Of the E. coli isolates, 18.8% (n = 9) were MDR. antimicrobial resistance can be resulted from extensively uses as growth promoters or therapeutically agents, leading to an emergency in the health field [34].
Also, the result was agreed with finding of Atoyebi, et al. [18] in which all the isolates (100%) of the isolated E.coli O157: H7 from table Eggs shell swab from Poultry Farms in Ibadan, Oyo State, Nigeria were multidrug resistant, that is they were all resistant to three or more tested antibiotics. The use of antimicrobials in food animals, as well as their role in promoting resistance in food-borne bacteria, is an important public health issue [26]. The association of various pathogenic bacteria in different diseases and selective pressure induced by accumulated antibiotic residue to develop antibiotic resistance is also emerging as the threat to human health [15]. Antibiotics are used in poultry farms to increase feed conversion ratio, to promote growth of birds, to prevent, treat and control diseases and to combats stress from environmental changes led to the emergence of antibiotics resistance strains as Antibiotics [36]. The effect of antimicrobial resistant genes in poultry and the possible transmission to human’s bacteria through animal foodborne and causes treatment failure in both animals and man leading to increased cost of treatment [18-29]. Unless all the necessary precautions are taken along the poultry production, marketing and processing chains, poultry meat and eggs can be contaminated by infectious agents that are harmful to humans (Table 5).
Poultry products can also be contaminated with the antimicrobial and anti-parasitic drugs or pesticides used on farms. The ingestion of antimicrobials can cause antimicrobial-resistant bacteria to develop in humans [11]. Bacteria can be exposed to these antimicrobial agents in nature and used for disease treatment, for prophylaxis, or for livestock growth promotion which can lead to resistance. Plasmid mediated with a wide variety of genetic determinants also contributes to resistance in these antimicrobials. This makes it more possible for a susceptible bacterium to acquire resistance factors through conjugation or transformation. Furthermore, the problem is probably associated with the widespread use of these antimicrobials in humans and animals for treatment of enteric infections [26]. The high prevalence of multidrug–resistant E. coli in table chicken egg shell supplied to the market center and shop from which the society can purchased for their food consumption, reflects a reservoir of resistance and easily transmission to humans as the they broken and cooking the internal content of eggs before they cleaning and disinfecting the shells If these resistance organisms to antimicrobial persist, there will be a great problem of antimicrobial choice in near future (Table 6).
The E. coli was recovered from eleven (11.95%) of the egg samples collected from the marketing centers and shop in Bishoftu town, East Shewa, Ethiopia. All isolates were multidrug resistance to more than two antimicrobial agents were detected in 11(100%) of the isolates in which the E. coli O157:H7 isolates resistance to five, and six antimicrobials. The ingestion of antimicrobials can cause antimicrobial-resistant bacteria to develop in humans. The raw table eggs provided to the markets for human consumption can contaminated with multiple drug resistance E. coli to antimicrobial agents commonly used in veterinary and human practice. Based on this conclusion the following recommendation was forwarded:
• Training programs must be provided on best practice of handling of eggs for handlers raising the level of awareness of people
• adoption of guidelines for the prudent use of antimicrobial agents in animals used for food.
• Improvement in the hygienic conditions of poultry farms and control of the misuse and overuse of antibiotics in poultry is therefore strongly recommended
• Consumers should be cleaning the egg shell before breaking for cooking
• Since E coli is resistant to most common drugs, attention should be taken in selecting antimicrobials in treating Salmonella infection both in animals and human being based on antimicrobial susceptibility test
• Further study ought to be conducted to identify the source of contamination
• The degree of the risk of consumption of egg contaminated with E coli should be assessed
• Education covering good sanitary practices for handling eggs to avoid cross-contamination or inadequate cooking is needed.


