Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Adel Badria*
Received: October 13, 2022; Published: October 20, 2022
*Corresponding author: Adel Badria, Department of Mechanical Engineering and Aeronautics, Division of Applied Mechanics, Technology of Materials and Biomechanics, University of Patras, Patras, Greece
DOI: 10.26717/BJSTR.2022.46.007398
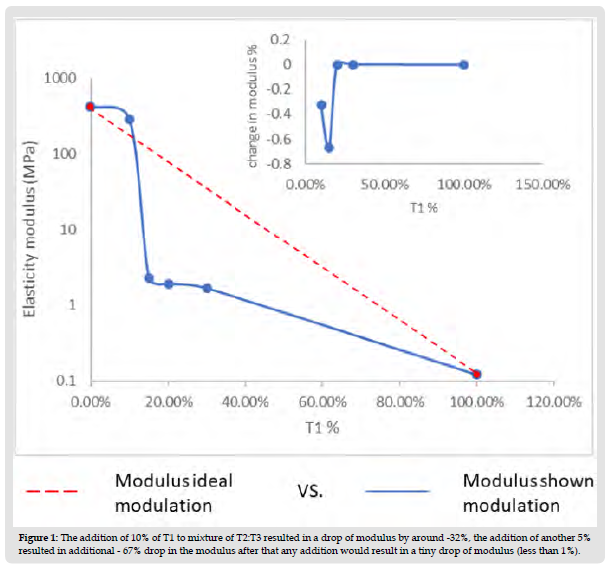
The use of thiol-ene chemistry in different fields has shown superiority over the common acrylate resin due to their oxygen insensitivity and relatively long gelation time which would lead to low internal stress in the final product. C.C. Cook, et. al. [1] reported a wide range of mechanical modulation for the different formulations of thiol:ene tested at fixed stoichiometric ratios. However, the relationship between the modulus of elasticity (E) and the different ratios of thiol:alkene1: alkene2 used shows a rough change instead of smooth modulus modulation. For example, upon addition of alkene2 to the thiol: alkene1at 10% ratio resulted in a -33% E drop, which changed to another -66% upon the addition of an extra 5% (total addition 15%). However, the further addition of alkene2 at 20%,30%,50% and 100% showed no more than a 1% decrease in E for each addition. Such observation leads us to believe that such modulation is partially attributed to incomplete reactions taking place due to the different reaction speeds between alkene 2 and 1 towards the thiol. C.C. Cook, et. al. [1] studied the degree of conversion using ATR-FTIR to confirm the completion of the different reactions. However, this ATR-FTIR was done only to two groups out of eight groups of different ratios of thiol:alkene1: alkene2. Such incomplete investigations caused an inaccurate conclusion. This comment is backed by a recent study of our group (https:// doi.org/10.1002/app.53046) as shown in this comment article.
Keywords: Polymerization; Polymer; Elastic Properties; Chemical Composition; Chemical Reaction; 3D Printing
The use of thiol-ene chemistry in different fields has shown superiority over the common acrylate resin due to their oxygen insensitivity and relatively long gelation time which would lead to a low internal stress in the final product (Ye, et al. [2]). Moreover, the ability to modulate the mechanical properties through having the right mixture of thiol-ene (s) compounds opened a wide door for their use in light-based 3D printing. In this journal C.C. Cook, et. al. [1] published a study for the usage of two alkene (T1, T2) and one thiol (T3) compounds to reach a wide range of elastic modulus modulation which ranged from 421 till 0.12 MPa (C.C. Cook, et. al. [1]). The authors argued that the complete stochiometric reaction taking place allowed for such modulation. In this comment we show that the mechanical modulation mentioned in C.C. Cook, et. al. [1] is partially due to an incomplete reaction between alkene and thiol. This conclusion is based mainly on two reasons:
1. The change in modulus of elasticity (E) upon the addition of 10% of the second alkene (T1) to the mixture of the alkene:thiol (T2:T3) resulted in initial sudden drop in E around -33% drop. Further addition of 5% resulted in another-66% drop in E. Such behavior was followed by less than 1% drop of E upon each further addition of 5%,10%, 20%, 50% and 100% as shown in (Figure 1). This could be interpreted as the fast gelation taking place between T1 and T3 trap the other alkene T2 not fully reacted in the mixture. Therefore, in Th_c sample the heat treatment allowed for the movement of the bulky alkene after the initial entrapment after UV treatment. However, at Th_d (which is only 5% T1 higher than Th_c), even with heat treatment the extent of gelation didn’t allow any further significant curing. This can be noticed in all the samples after Th_c.
Figure 1 The addition of 10% of T1 to mixture of T2:T3 resulted in a drop of modulus by around -32%, the addition of another 5% resulted in additional - 67% drop in the modulus after that any addition would result in a tiny drop of modulus (less than 1%).
2. In a recent published work of our lab (Badria, et al. [3]), we exploited the same concept and compounds T2 and T3. However, instead of using two alkene and one thiol compounds we used two thiols (T3, Tx) and one alkene (T2) (Figure 2). The Tx thiol used was pentaerythritol tetrakis(3- mercaptopropionate) which is a tetrathiol noncyclic which is less bulky and more thiol functional groups than the other thiol (T3). We faced the same sudden drop upon addition of the second thiol to the mixture. However, repeating the same experiment in the presence of different concentrations of photoabsorber showed a significant difference (higher modulus; 400 and 600 Mpa depending on the photabosorber concentration) between the thiol:thiol:ene mixture with and without this photoabsorber. We attributed this result to the retarding effect of photoabsorber for the reaction (gelation) allowing a higher chance for the bulky compounds to move through the mixture solution and react at higher percentages. For a further confirmation of our hypothesis (the differential reaction speed between the different thiol towards the alkene), the curing speed and penetration depth of the different mixtures were measured at certain laser light energy. The results showed that Tx: T2:T3 has the lowest curing speed Tx: T2:T3< T2:T3< T2: Tx. Bringing together these two experiments, we found that the tetrathiol noncyclic thiol Tx requires less energy for curing which led to a faster curing and gelation in comparison to the bulky tricyclic T3. Under high energy radiation this leads to a cured product of T2: Tx where T3 is physically trapped unreacted in the mixture which leads to the sudden drop in E (not gradual). On the other hand, the lowering of the laser energy using photoabsorber delays enough the gelation time and penetration depth of the Tx, allowing bulkier T3 to move within the solution giving a final higher E.
Conclusively thiol-ene chemistry could offer a great option in the area of mechanically modulated polymers. However, the selection of the correct combination of different thiols and alkenes should take into consideration the curing speed between the different compounds involved in the final reaction.
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statements
The author declares that the data supporting the findings of this study are available within the article.


