Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ahmet Ozgun DOGRAMACI, Cemile OZCAN*
Received: Navember 03, 2022; Published: November 09, 2022
*Corresponding author: Cemile OZCAN, Department of Chemistry, Science and Art Faculty, Kirklareli University, Kırklareli, Turkey
DOI: 10.26717/BJSTR.2022.47.007447
The aim of this study, for determination of verteporfin in real samples (simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water) was the method validation and to examined by HPLC-DAD-UV. Metod validation parameters such as specificity, linearity, precision, accuracy, robustness, limit of detection (LOD) and limit of quantitation (LOQ) for verteporfin were validated and developed according to the International Conference on Harmonization (ICH) Q2 R1 guidelines. The LOD and LOQ for verteporfin were found 0.06 μg/L and 0.2 μg/L, respectively. The recovery values of the optimization and validation for verteporfin were found in the range of 97.5-100.7%. The relative standard deviations (RSD) for vertepofin were <1%. The developed method was successfully applied to real samples with high accuracy and the recoveries (%) from real samples were 99.9, 100, 98.2, 99.2, 99.4, 98.8 and 99.4, respectively.
Keywords: HPLC-DAD-UV; Verteporfin; ICH Q2 R; Validation
Abbreviations: LOQ: limit of Quantitation; LOD: limit of Detection; RSD: Relative Standard Deviations; TEAD–YAP: Transcriptional Enhanced Associate Domain–Yes Associated Protein; CE: Capillary Electrophoresis; HPLC-DAD/UV: High Performance Liquid Chromatography-Photodiode Array Detector/Ultraviolet; ICH: International Conference on Harmonization; CF: Capacity Factor
Verteporfin (Figure 1) is a benzoporphyrin derivative used clinically a second-generation porphyrin-derived photosensitizer for photodynamic therapy of age-related macular degeneration [1-3]. Verteporfin has two regioisomers, BPD-MAC and BPD-MAD and are present in a 1:1 ratio and the potentials of the isomers (cis and trans) are equal [4,5]. Verteporfin is a small molecule that inhibits transcriptional enhanced associate domain-Yes Associated Protein (TEAD-YAP) association and YAP-induced liver overgrowth [6]. Verteporfin is an autophagy inhibitor that blocks autophagy at an early stage by inhibiting autophagosome formation [7,8]. Verteporfin inhibits cell proliferation and induces apoptosis [8]. Red light irradiation gives rise to the generation of oxygen radicals that nonselectively kill cells exposed to verteporfin. Moreover, verteporfin is a very hydrophobic drug and displays little or no cellular toxicity in the absence of light activation [7,9].
Verteporfin was approved for the treatment of age-related macular degeneration [10,11]. The potential of verteporfin for the treatment of cancers, such as prostatic cancer, breast cancer, and pancreatic ductal adenocarcinoma has been investigated [12,13]. Since verteporfin is a lipophilic molecule, it is rapidly taken up by tumor cells and rapidly dividing active cells [14]. Liposomal verteporfin, which is hydrophobic, present generally amphiphilic properties due to structures contain hydrophilic and lipophilic regions, can dissolve in both water and fat [15]. Verteporfin also has been reported to inhibit autophagy at an early stage by suppressing autophagosome formation [7]. In addition, Gu et al. observed that protoporphyrin IX and verteporfin exhibited potent antiviral activity and prevented SARS-CoV-2 infection [16]. The absorption spectrum range of verteporfin is between 350 and 695 nm, giving maximum absorption at wavelengths of 354, 418, 430, 574, 626, 680, 687, 690, 692 and 700 nm in different organic solvents [3,10,17].
It has been reported to be a potent agent for Photodynamic Therapy when activated by laser light of 689 nm wavelength [9]. In addition, it is recommended to use a 26% solution (prepared in 5% dextrose solution) for the daily intake dose of 6 mg/m2 [18,19]. The extensively used and highly sensitive analytical techniques for determination and quantification of verteporfin are LC-MS [9], capillary electrophoresis (CE) [20] and UV [17]. Besides being a chromatography technique, HPLC produces results with high efficiency and resolution. In this study for verteporfin (cis (I) and trans (II)) active ingredient, a benzoporphyrin derivative, is aimed to (i) development-validation-optimization as an alternative method in HPLC, (ii) the recovery studies (iii) determination in real samples (simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples). Because HPLC is the most widely used analytical separation technique for the qualitative and quantitative determination of compounds in natural product extracts.
Materials
The verteporfin I and II was analyzed by high performance liquid chromatography-photodiode array detector/ultraviolet (HPLC-DAD/UV), using a Shimadzu LC-2030C and LC-2030C 3D Plus system. In the preparations of the standards and samples were used ultrasonic bath (Bandelin RK 1028 H model).
Standards and Reagents
The standard of verteporfin (Lot R049D0, Cas Number: 129497-78-5, 96.8%) were purchased from USP reference standard. All solvents were HPLC-grade (Merck and JT Baker) and all other chemicals were analytical reagent grade. Double-distilled water (HPLC-grade (18.2 MΩ)) for all preparations obtained was used through a purification system (ELGA). The mobile phase was prepared by mixing acetonitrile (ACN):tetrahydrofuran (THF):buffer solution:acetic acid (AA) (11:9:20:2, v:v:v:v) ratios. While preparing 0.025 M ammonium acetate buffer, the pH was adjusted to 3.0 ± 0.05 with 3.6 M sulfuric acid and mixed in an ultrasonic bath. Filtered through a 0.45 μm membrane filter, the amber colored bottle was made ready for use and prepared fresh daily before analysis. The standard stock of verteporfin was diluted with the mobile phase to obtain the calibration standards at concentrations of 8, 9, 10, 11 and 12 mg/L verteporfin. The peak areas were plotted against the corresponding concentration to obtain the calibration graph. Calibration curve and correlation coefficient (R2) were calculated by least squares linear regration analysis. This method was validated according to the International Conference on Harmonization (ICH) Guideline and Text on Validation of Analytical Procedures [21-30].
Optimization of HPLC-DAD-UV for Vertepofin
For the optimization, the flow rate (1.8 mL/min, 2.0 mL/ min, 2.2 mL/min ) of the mobile phase, injection volume, column temperature (35, 40, 45 °C) and wavelength were examined. In the each studied parameter, the other parameters were chosen at optimum value by using verteporfin solution of 10 mg/L.
Validation Procedure
The novel developed HPLC method was validated according to the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) [27]. This method was validated regarding linearity, precision, specificity, accuracy, robustness, limit of detection (LOD), and limit of quantification (LOQ) from minimum 6 replicates and 6 parallel for verteporfin. The precision of method was expressed as standard deviation and RSD% of standard measurements. Precision was measured using a minimum of six determinations (replicates) per concentration. The concentration levels (containing to range from low, medium and high) in the range of the expected concentrations was investigated to proximity of measurements.
Linearity
Linearity was analyzed at six different concentrations ranging from 5 mg/L to 12 mg/L. Each concentration was injected six times in order to obtain the area under the curve which corresponded to each concentration. Accordingly, the area under the curve data were plotted versus verteprofin I and II concentrations (mg/L), separately. Linear regression analysis was assessed to determine the calibration equations [21-30]. Calibration equations were expressed as y= ax+b, for which a and b coefficients represent the slope and intercept of the curves, respectively. An six level (5-12 mg/L for verteporfin I and II isomers) calibration series were established with six analyses at each concentration level for determining linearity.
Accuracy
Three replicates of 5, 8, 10, and 12 mg/L concentrations were analyzed to determine the closeness of the obtained results with the actual amounts. Accuracy was reported as recovery and the relative standard deviation (RSD%) for each concentration.
Precision
Intraday and interday precisions are regarded as the major parameters for a validated analytical method. Intraday precision was conducted by analyses of samples with concentrations of 5, 8, 10, and 12 mg/L in replicates of six (every 1 hours). Interday precision was assessed by analyzing various concentrations (5, 8, 10, and 12 mg/L) in six replicates for three consecutive days. Both intraday and interday precisions were reported as the mean measured concentration along with the relative standard deviation (RSD).
Limit of Detection (LOD) and Limit of Quantification (LOQ)
The verteprofin standard mixture solution was diluted to determine the LOD and LOQ. The LOD and LOQ are defined as the minimum concentration which possesses a signal-to-noise ratio by using the 3 Sb/m and 10 Sb/m values, respectively [21-30].
Robustness and Ruggedness
As recommended by ICH guidelines, a robustness assessment was performed for the development of the validated analytical method [27]. Robustness indicates the ability of a method to tolerate small deliberate changes in the flow rate and mobile phase composition. Briefly, the flow rate was set at 1.8, 2.0, and 2.2 mL/min and the recoveries of verteporfin were analyzed as a response. The mobile phase stability and the effect of column temperature change (35, 40 and 45 °C) was another parameter which was analyzed in the robustness study. Furthermore, some minor modifications in temperature and detection wavelength were applied. A ruggedness study was also carried out which confirms that the method is reproducible by different analysts.
Stability Studies
The stability of samples was analyzed at two different temperatures. Verteprofin I and II (QC samples) were kept at refrigerator (2–8°C) and room temperature. After 3 hours, the samples were injected onto the HPLC system for further analysis. The concentrations of all samples were 15 mg. The results of the stability studies were reported as the recovery perentage for verteporfin. The recovery rates were determined by adding 50- 100-200 μg L-1 concentration standards for samples. The matrixes used for recovery studies were used for simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples, respectively (showed in Table 2). This method was validated regarding linearity, precision, specificity, accuracy, robustness, limit of detection (LOD), and limit of quantification (LOQ) from minimum 6 replicates and 6 parallel for verteporfin.
Preparation of Real Samples for Analysis of Verteporfin
The stock solution was firstly prepared which for investigation of verteprofin in simulated body fluid, simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples. For this, one vial containing Lyophilized Powder for Verteporfin Solution was weighed as full and dissolved with the mobile phase solvent, and a 150 mL amber-colored balloon was transferred into a jug and kept in an ultrasonic bath for 2 minutes, and the stock solution was prepared by dissolving. At room temperature, it was made up to the final volume with solvent. 1.0 mL was taken from the stock solution with a glass pipette, transferred to a 10 mL amber-colored volumetric flask, completed to its volume with real samples, and vortexed. The solution of the real samples prepared was filtered through a syringe filter of 0.45 μm (PTFE) and afterwards, analyzed by HPLC-DAD.
Statistical Analysis
The whole data were subjected to a statistical analysis and correlation matrices were produced to examine the interrelationships and investigated verteprofin in simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples.
The obtained optimum conditions and HPLC-UV-DAD parameters were given in Table 1. For validation purposes, a blank verteporfin-free was selected and different validation parameters were evaluated, including linear range (linearity), recovery, precision and method LOD and LOQ according to the International Conference on Harmonization (ICH) Q2 R1 guidelines [29,30]. Calibration graphs for verteporfin were established in a range of 5-12 mg/L with correlation of coefficients from 0.9996 (R2) to 0.9998 (r) for verteprofin (Figure 2). The retention time (RT min), linear regression (y=ax+b), the determination coefficient (r2), LOD, LOQ and %RSDs are shown in Table 2. Linear regression equation drawn by HPLC was applied and used for quantification. This method was validated by determining of verteporfin recovery from simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples by applying the procedure.
Table 2: The Retention time, linear regression, correlation coefficients, LOD, LOQ and RSD% for verteporfin.
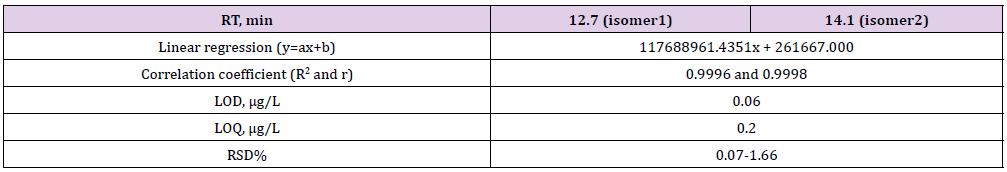
LOD and LOQ ranged from 0.06 to 0.2 μg L-1 for verteporfin. The relative standard deviations (RSD %) determined for the method and injection repeatabilities were less than <1% for verteporfin, indicating the good repeatability of the method. The more linear trans isomer has more interactions with the C18 stationary phase and elues after corresponding cis isomer [9]. As part of the robustness, deliberate change in the flow rate, mobile phase stability and column temperature of ±10% was made to evaluate the impact on the method. The results showed that the method is robust (Table 3-5). The capacity factor (CF) is not affected by the flow rate. Because, the CF remains constant while the retention times change with the change in flow rate. The capacitance factor is the parameter controlled by changing the power of the mobile phase and has a great effect on the resolution. If the resolution approaches zero at low output times the separation of isomer peaks cannot be achieved. As the capacity factor (CF) increases, the resolution increases and when the CF is between 2-10, good resolution is provided. In our study, good resolution was obtained for the variation of flow rate, mobile phase stability and column temperature for verteporfin.
For in-laboratory precision of verteporfin analysis in the LPVV solution sample, two different analysts which using different days and equipment studied, the results are given in the Table 6. Furthermore, the reliability of the method was tested using the student-t test. RSD% for intraday and interday assays ranged from 0.02% to 0.7% and 0.16% to 0.32%, respectively (Table 7). The optimized conditions for HPLC-DAD were found to be 2 mL min−1 for flow rate, 50 μL for injection volume, column temperature 40 °C, 410 nm wavelength and chosen as 5 °C the sample chamber temperature. This method was applied for the determination of verteporfin in simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water samples. The contents of the simulated liquids are given in the Table 8 The recoveries of the vial sample containing lyophilized powder for verteporfin solution in prepared simulated liquids (simulated body fluid and simulated tears), liquids used in intravenous applications (0.9% isotonic sodium chloride solution, Lactated Ringer’s I.V. solution for infusion and 5% dextrose solution for I.V. infusion in water), lemon juice and drinking water were examined and were 98.2-100%, with RSD% less than 2.0%, shown in Table 9.
HPLC-DAD method for estimation of verteporfin in their pharmaceutical dosage form and real samples was established and validated as per the ICH guidelines. Linearity, sensitive, rapid, precise, rugged, accurate, specific and robust was achieved for verteporfin and isomers. Moreover, the obtained results were found lower than in the literature described Table 10.
This study describes a more sensitive and reliable method for identification and quantitative analysis of verteporfin were developed and validated by HPLC-DAD method. The method was validated by recovery studied for linearity, robustness, precision, accuracy and repeatability. The newly developed method showed acceptable precision and accuracy at least in the concentration range of 5 to 12 mg/L. The developed method was successfully applied for the quantification and chromatographic separation analysis, which represented good resolution for verteprofin. The validated analytical method is simple and reproducible which can be used in quality control departments. The applicability of this method to determine verteporfin in real samples (simulated body fluid, and simulated tears, 0.9% isotonic sodium chloride solution, Lactated Ringer IV solution for infusion, 5% dextrose IV solution for infusion, lemon juice and drinking water) was demonstrated as well as providing an appropriate detection limit and good recovery and demonstrating a wide linear range.
This work was supported by the Kirklareli University Scientific Research Projects Coordination Unit. Project Number: KLUBAP-11/-12/-120/-121/-198 and /-235.
Conflict of interest the authors declare that they have no conflict of interest.


