Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mohamed Ahmed1* and Ahmed Shoker2
Received: June 15, 2023; Published: June 26, 2023
*Corresponding author: Mohamed Ahmed, Assistant Professor of Anatomy, Department of Basic Sciences, California Northstate University, West Taron Drive, Elk Grove, CA, USA
DOI: 10.26717/BJSTR.2023.51.006053
Background and Objectives: Nebivolol is a selective ß1- adrenergic antagonist with endothelial and nitric oxide- (NO) dependent vasodilation properties. Tacrolimus (Tac) increases the risk of diabetes mellitus. We investigated whether nebivolol reverses the deleterious effect of Tac on the pancreas in Sprague Dawley Rats.
Materials and Methods: Animals were subdivided into 4 groups of six animals each. group 1, control, received 0.5 mg/kg/day of castor oil (s/c), group 2 received 1.5 mg/kg/day of Tac (s/c), group 3 received 1 mg/kg/day of nebivolol (s/c), and group 4 received Tac {1.5 mg/kg/day of Tac (s/c)}and nebivolol {1 mg/ kg/day of nebivolol (s/c)}. Insulin was measured by ELISA and insulin resistance (IR) was calculated from HOMA-IR. Blood flow was measured by conventional microsphere method. The duration of the study was 45 days.
Results: Pancreatic blood flow measurements in groups 1, 3 and 4 were similar. Flow in group 2 (0.08±0.02 ml/g) was significantly lower than that in groups 4 (1.1±0.4 ml/g), group 1 (0.6±0.2 ml/g), and group 3 (0.9±0.3 ml/g) (p<0.05). Fasting plasma glucose (FPG) levels in groups 1, 3 and 4 were similar. FPG levels in group 2 (7.3±0.1 mmol/L) were significantly higher than those in groups 1 (6.6±0.1 mmol/L), group 3 (5.7±0.24 mmol/L) and group 4 (5.70±0.21 mmol/L) (p<0.05). Plasma insulin levels in group 2 (0.069±0.02 μg/L) were much lower than those in groups 1 (0.22±0.06 μg/L), group 3 (0.268+- 0.2 μg/L) and group 4 (0.195±0.09 μg/L) (p<0.05). HOMA- IR values were similar.
Conclusion: Nebivolol enhances pancreatic blood flow, reverses hyperglycemia, and normalizes insulin secretion in animals treated chronically with high dose Tac.
ß-Adrenergic receptor antagonists play an important role in the management of cardiovascular disease, including hypertension, coronary artery disease and chronic heart failure [1]. Traditional ß- blockers are no longer first- or second- line antihypertensive therapy until third- generation ß-blockers with ß1- adrenergic receptor selectivity was emerged as an effective therapy for hypertension [2-9], heart failure and left ventricular dysfunction [10,11]. The second major mechanism of action of nebivolol is nitric oxide-(NO)-mediated vasodilatation [12-16]. This is likely to be mediated by stimulation of ß3-adrenergic receptors [12,17-20]. These receptors have been traditionally related to metabolic effects of sympathetic stimulation (lipolysis in adipocytes, insulin sensitivity) [1]. Several studies suggest that the ß3-adrenergic stimulation could be a new therapeutic target for the treatment of cardiovascular diseases [21]. The vascular effects induced by ß3-adrenergic stimulation can decrease the left ventricular strain allowing the reduction of after-load [21]. In addition, the increased coronary blood flow due to vaso-relaxation increases the myocardial oxygen delivery [21].
Regarding metabolic issues, it was demonstrated that first- and may be second- generations of ß blockers elicit a diabetogenic effect through a mechanism involves decrease in pancreatic insulin secretion and/or release secondary to a reduced peripheral blood supply [22]. Increased body weight and gluconeogenesis through glycogenolysis secondary to unopposed α2 activity are also possible fates [22]. Not a surprise that the term “new onset diabetes’ (NOD) meaning new arisen diabetes due to antihypertensive therapy is closely related to classical ß blockers and some other therapies [22]. A large meta-analysis studied the prevalence of NOD in hypertensive population treated with ß blockers [23]. ß blockers resulted in approximately 30% increased risk of NOD as compared to placebo and 20% increased risk when compared to calcium antagonists and RAAs antagonists [22]. However, comparing against thiazide diuretics revealed a neural [24,25] to less diabetogenic effect of ß blockers [23] (Figure 1).
However, the effect of nebivolol on insulin resistance can be partially attributed to its ability to significantly improve endothelial dysfunction [26]. This improvement may lead to a reduction in insulin resistance and vice versa [26,27]. Furthermore, nebivolol is an agonist of endothelial beta-3 adrenoreceptors, which also play a key role in glucose homeostasis and lipid metabolism [26]. Nebivolol, in contrast to conventional beta-blockers such as metoprolol, improves oxidative stress, decreases plasma-soluble P-selectin, and increases adiponectin levels in hypertensive patients [28]. Tacrolimus (TAC), a macrolide antibiotic produced by Streptomyces tsukubaensis is an immunosuppressant belongs to calcineurin inhibitors drug class. TAC is used to prevent rejection after solid organ transplantation. One of the most adverse effects of TAC administration is post-transplant diabetes mellitus (PTDM) [29]. It was thought that the main mechanism underlying the diabetogenic effect of TAC encompasses both impaired insulin secretion secondary to islet ß-cell toxicity [30] and insulin resistance [29]. It seems that pancreatic toxicity is dose-dependent [29]. Additionally, several studies have been conducted on rats and mice to highlight the other possible mechanisms that might lead to PTDM. One of the causes that might lead to PTDM is diminishing pancreatic blood flow [31] and/or islet blood flow [31-34] (Figure 2).
Reducing skeletal muscle blood flow, however, could be another factor that might contribute to diabetes mellitus. Baron introduced the novel concept that insulin could regulate its own delivery, and that of glucose, by increasing blood flow to muscle [35]. Insulin acts on the skeletal muscle vasculature at three discrete steps to enhance its own delivery to muscle:
Relaxation of resistance vessels to increase total blood flow.
Relaxation of pre-capillary arterioles to increase the micro-vascular exchange surface perfused within skeletal muscle (micro-vascular recruitment) and
The trans-endothelial transport of insulin [36]. Insulin can relax resistance vessels and increase blood flow to skeletal muscle [36]. Based on that, we thought that the disturbance in insulin release and sensitivity might be due to reducing pancreatic blood flow and might lead to impaired glucose uptake by skeletal muscles which may be a possible cause of hyperglycemia.
Animals and Drugs
Twenty four S/D rats were subdivided into 4 groups, group 1 control (n=6), received 0.5 mg/kg/day of castor oil (Sigma Aldrich, ON, Canada) (s/c), group 2 (n=6), received 1.5 mg/kg/day of TAC (Astellas Pharma Inc, Canada) (s/c), group 3 (n=6), received 1 mg/kg/day of nebivolol (Sigma Aldrich, ON, Canada) (s/c), and group 4 (n=6), received 1.5 mg/kg of TAC (s/c) + 1 mg/kg/day of Nebivolol (s/c). The duration of the study was extended up to 45 days.
Body weight
The body weights of the animals were recorded daily throughout the period of the study.
Microsphere Preparation
Before the surgical procedure began, a microsphere solution was prepared, vorticed for 15-30 seconds and sonicated for 3-5 minutes.
Surgical Procedures
Rats were anaesthetized with inhalation using isoflurane. Animals were placed on heating pad to maintain body temperature at 37°C and allowed to breathe by inserting a tracheal tube. Right femoral artery was cannulated with PE-50 tubing (connected to needle and syringe and filled with heparinized saline) and connected to a pressure transducer for the measurement of mean arterial pressure and heart rate, which is connected to Power Lab Data Acquisition System. The left femoral artery was cannulated with PE-50 tubing and connected to reciprocal syringe pump for the collection of reference blood samples when microspheres are injected with saline and heparinized saline. The right carotid artery was cannulated to the left ventricle for injecting microspheres (Figure 3).
Microsphere Injection
Yellow-green and red microsphere solutions were vorticed, immediately infused into the left ventricle for a period of 20 seconds, and simultaneously saline was infused through the left femoral vein. Carotid cannula was flushed with saline for 80 seconds to ensure that no microspheres remain in the carotid artery. Reference blood sample was collected for 80 seconds from femoral artery and immediately transferred in pre-weighted 15mL glass tube and placed it on ice.
Tissue Collection and Processing
Organs were quickly removed, collected, cleansed, dried, and transferred to a 15 mL pre-weighed glass tube and weighed. Ten mL ethanolic KOH (4M) solution per gram tissue were added and kept in shaker water bath (Forma Scientific) at 37°C for 2-4 days. Vorticed homogenized samples were centrifuge at 3400 RPM for 20 minutes and carefully and supernatant was removed with suction system leaving 1ml remaining. Nine ml of 0.25% Tween 80 in saline were added vorticed and centrifuged twice at 3400 rpm for 20 min before careful removal of supernatant with suction system leaving 1 ml remaining. Nine ml K+ PBS in triple distilled water were added, vorticed and centrifuged at 3400 rpm for 20 minutes. Supernatant was carefully removed with suction system leaving 1 ml remaining. Two ml cellusolve were added, vorticed and let rest for 2-8 hours prior to centrifuging for 15 minutes at 3400 rpm. One hundred μl of supernatant were pipetted (should be separated clearly) into individual wells of a 96 well microplate in triplicate for each sample. The fluorescence intensity of each well was measured at excitation and emission wavelengths specific to the microsphere’s use. Files were saved in Excel™ and standards were checked for consistency (Figure 4).
Biochemical Testing
Fasting plasma glucose, insulin as well as blood levels of FK506 (TAC) were measured in Chemistry Lab using an automated Synchron LX20 Clinical System Analyzer (Beckman Coulter Inc, USA).
Homeostasis Model Assessment of Insulin Resistance (HOMA-IR)
HOMA-IR was calculated from fasting plasma glucose (mmol/L) and fasting plasma insulin (µg/L).
Statistical Analysis
Results were presented as mean ± standard error. Statistical comparisons were performed using the Prism graph-pad. Unpaired T-test and F-test were used for comparing hemodynamic as well as biochemical parameters among groups. P-values less than 0.05 are considered significant.
Hemodynamic Changes Experiments
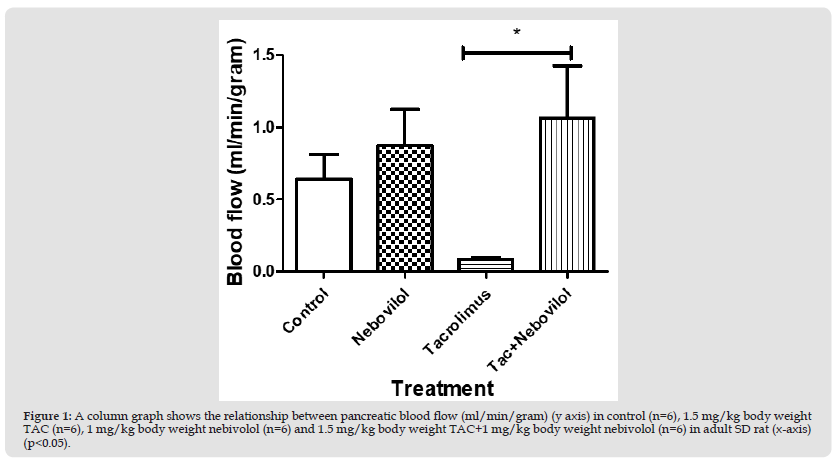
First Experiment: Pancreatic blood flow measurements (ml/g) revealed insignificant differences between control, group 1 (0.5 mg/kg/day castor oil) (0.6±2 ml/g) vs. group 3 (1 mg/kg/day nebivolol) (0.9±0.3 ml/g) and group 4 (1.5 mg/kg of TAC+1 mg/kg/day nebivolol) (1.1±0.4 ml/g). Blood flow measurements in group 2 (0.08±0.02 ml/g) were significantly lower than those in groups 4 (1.1±0.4 ml/g), group 1 (0.6±0.2 ml/g), and group 3 (0.9±0.3 ml/g) (p<0.05).(Figure 1)
Figure 1 A column graph shows the relationship between pancreatic blood flow (ml/min/gram) (y axis) in control (n=6), 1.5 mg/kg body weight TAC (n=6), 1 mg/kg body weight nebivolol (n=6) and 1.5 mg/kg body weight TAC+1 mg/kg body weight nebivolol (n=6) in adult SD rat (x-axis) (p<0.05).
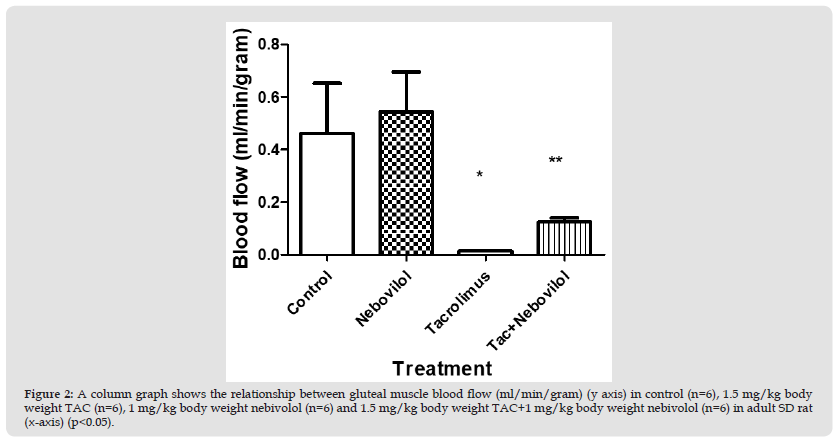
Second Experiment: Gluteal muscle blood flow measurements (ml/g) revealed a significant difference between TAC treated group (1.5 mg/kg Bwt) (0.01±001 ml/g) and TAC + nebivolol treated group (1.5 mg/kg of TAC+1 mg/kg/day of nebivolol) (0.1± 0.01 ml/g) (p<0.05). However, values from controls (0.5±0.1 ml/g) and nebivolol treated group (1 mg/kg Bwt) (0.5±0.1 ml/g) were insignificantly different.(Figure 2)
Figure 2 A column graph shows the relationship between gluteal muscle blood flow (ml/min/gram) (y axis) in control (n=6), 1.5 mg/kg body weight TAC (n=6), 1 mg/kg body weight nebivolol (n=6) and 1.5 mg/kg body weight TAC+1 mg/kg body weight nebivolol (n=6) in adult SD rat (x-axis) (p<0.05).
Third Experiment: Diaphragmatic muscle blood flow measurements (ml/g) revealed a significant difference between TAC treated group (1.5 mg/kg Bwt) (0.1±03 ml/g) and TAC + nebivolol treated group (1.5 mg/kg of TAC+1 mg/kg/day of nebivolol) (0.4± 0.1 ml/g) (p<0.05). However, values from controls (0.4±0.1 ml/g) vs. nebivolol treated group (1 mg/kg Bwt) (0.5±0.0.09 ml/g) and TAC+nebivolol treated group (0.4± 0.1 ml/g) were insignificantly different.(Figure 3)
Figure 3 column graph shows the relationship between diaphragmatic muscle blood flow (ml/min/gram) (y axis) in control (n=6), 1.5 mg/kg body weight TAC (n=6), 1 mg/kg body weight nebivolol (n=6) and 1.5 mg/kg body weight TAC+1 mg/kg body weight nebivolol (n=6) in adult SD rat (x-axis) (p<0.05).

Biochemical Parameters
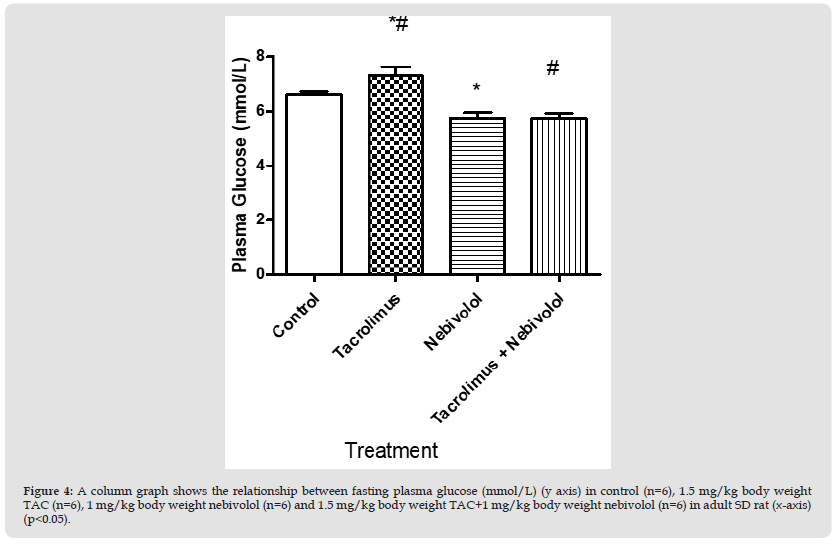
Fasting Plasma Glucose: Fasting plasma glucose (mmol/L) revealed significant differences between group 2 (7.3±1 mmol/L) (n=6) vs. group 4 (5.70±0.21 mmol/L) (n=6) and group 3 (5.7±0.24 mmol/L) (n=6) (p<0.05). Fasting plasma glucose levels in group1 (6.6±0.1 mmol/L), group3 (5.7±0.24 mmol/L) and group 4 (5.70±0.21 mmol/L) were insignificantly different.(Figure 4)
Figure 4 A column graph shows the relationship between fasting plasma glucose (mmol/L) (y axis) in control (n=6), 1.5 mg/kg body weight TAC (n=6), 1 mg/kg body weight nebivolol (n=6) and 1.5 mg/kg body weight TAC+1 mg/kg body weight nebivolol (n=6) in adult SD rat (x-axis) (p<0.05).
Fasting Plasma Insulin: Plasma insulin levels (µg/L) revealed a significant difference between group 2 (0.069±02 µg/L) vs. group 1 (0.22±0.06 µg/L), and group 4 (0.195±0.09 µg/L) (p<0.05). However, group 3 (0.268+- 0.2 µg/L) revealed no significant differences with group 2 (0.069±0.02 µg/L) vs. group 4 (0.195±0.09 µg/L). Also, no significant difference was revealed between group 1 (0.22±0.06 µg/L), and group 4 (0.195±0.09 µg/L).(Figure 5)
Figure 5 A column graph shows the relationship between fasting plasma insulin (μg/L) (y axis) in control (n=6), 1.5 mg/kg body weight TAC (n=6), 1 mg/kg body weight nebivolol (n=6) and 1.5 mg/kg body weight TAC+1 mg/kg body weight nebivolol (n=6) in adult SD rat (x-axis) (p<0.05).

Homeostasis Model Assessment of Insulin Resistance (HOMA-IR): HOMA-IR revealed no significant differences among all groups.
The diabetogenic effect of ß- adrenergic antagonists is likely to be class specific. Non-selective 1st generation (propranolol, sotalol) and ß1- selective 2nd generation ß-receptor antagonists (metoprolol, atenolol) have been found to reduce insulin sensitivity and secretion [37,38]. Eleftheriadou et al suggested that the reduction of insulin sensitivity is attributed to decreased cardiac output and thus to reduced blood flow to skeletal muscles while the reduction of insulin secretion is likely due to blockade of ß2-pancreatic receptors [37]. On the other hand, 3rd generation ß- receptor antagonists (nebivolol, carvedilol) exert a positive potential effect on insulin sensitivity and secretion. Carvedilol elicits its action by the blockade of α1- receptors that results in peripheral vasodilation and thus to increase peripheral blood flow and enhancement of insulin sensitivity [39]. Nebivolol, however, as a ß1-selective receptor antagonist, possesses a nitric oxide (ON) mediated vasodilatory effect and an antioxidant favorable effect on both carbohydrate and lipid metabolism [40]. Stoschitzky et al. have demonstrated similar effects of carvedilol and nebivolol on heart rate and blood pressure in healthy volunteers [41,42]. However, according to them, nebivolol treatment has been associated with significantly better quality of- life outcomes compared with carvedilol (p < 0.05) [42]. Furthermore, compared with carvedilol, nebivolol has been shown to exert a greater inhibitory effect on platelet aggregation [43]. Because platelets are directly implicated in the pathogenesis of vessel wall complications in hypertensive patients, nebivolol may be a safer choice in this setting [44] (Figure 5).
In our study, we have indicated that tacrolimus impaired glucose homeostasis. Hemodynamic studies indicated a decrease in pancreatic and skeletal muscles (gluteal and diaphragmatic) blood flow (ml/min/g) when the results from tacrolimus-treated rats’ group were compared against tacrolimus- as well as tacrolimus + nebivolol-treated groups (p<0.05). However, fasting plasma glucose (mmol/L) was higher in tacrolimus group when compared with tacrolimus + nebivolol treated group (p<0.05). Furthermore, fasting plasma insulin (µg/L) was lower in tacrolimus group when compared with tacrolimus + nebivolol treated group (p<0.05).
Pancreatic Blood Flow
Based on our findings, we thought that treating SD rats with tacrolimus (1.5 mg/kg body weight) for 45 days might exert its diabetogenic potential effect through decrease in pancreatic blood flow. This was correlated with the findings of Ito et al. and Hernandez-Fisaca, et al. [45,46] as well as a previous study has been conducted by us [47], using the same dose of tacrolimus (1.5 mg/kg body weight) but for a shorter period compared to the current study (21 days). We and others [45,48] thought that tacrolimus reduces pancreatic and/or islet blood flow in a dose-dependent manner in acute and chronic studies.
Skeletal Muscle Blood Flow
Several pre- and clinical studies have shed light on possible treatment options of anti-hypertensive agents in pre- and diabetic normal and hypertensive volunteers over the last two decades. RAAS antagonists have been used extensively in pre-clinical and in clinical sittings to amplify their role in inducing insulin release and/or sensitivity through enhancing pancreatic blood flow. Huang, et al. [49] described the effect of intravenous injection of 3 mg/kg body of irbesartan and 3 mg/kg body weight of captopril into anaesthetized female Wistar rats. Blood flow rates were determined by a microsphere technique [49]. They concluded that pancreatic blood flow was markedly increased by captopril (P<0.05) and irbesartan (P<0.01) [49]. Pancreatic islet blood flow was significantly and preferentially enhanced after the administration of captopril (P<0.01), irbesartan (P<0.01) [49]. The overall finding is that these vasoactive drugs augments insulin secretion and improves glycemia by enhancing pancreatic blood flow. Also, Chan, et al. [50] described the effect of Ang II receptor antagonist, valsartan on the plasma glucose and insulin levels in streptozotocin-induced diabetic rats. They found that valsartan effectively lowered plasma glucose and this was associated with an increase in the glucose utilization in peripheral tissue and/or a reduction in hepatic gluconeogenesis in the absence of insulin [50]. In the same essence, Ang II has been shown to adversely influence pancreatic and/or islet blood flow through vasoconstrictive effects [51,52] and this effect was found to block glucose stimulated insulin secretion, an event fully reversible by losartan [51,52]. In clinical sitting, RAAS antagonists showed that blockade of RAAS by an angiotensin-converting enzyme inhibitor (ACEI) and/or an angiotensin II receptor blocker (ARB) decreased adipocyte size with improvement in insulin sensitivity [53,54].


