Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yasmeen Sultana, Sharmin Musa and Hamida Khanum*
Received: August 14, 2023; Published: August 21, 2023
*Corresponding author: Hamida Khanum, Department of Zoology, University of Dhaka, Dhaka, Bangladesh
DOI: 10.26717/BJSTR.2023.52.008238
The present study was conducted to investigate the endo-parasite infestation in Xenentodon cancila and Polynemus paradiseus during January 2017 to December 2018. A total of 9 species of parasites were collected and identified from X. cancila, of which two were trematodes (Bolbocephalus sp, Isoparorchis hypselobagri); four nematodes (Metaquimperia bagari, L3 larva of Gnathostoma spinigerum, Camallanus ophiocephali, Porrecaecum trichuri.) and three acanthocephalans (Neoechinorhynchus prolixum, Acanthocentis nigeriensis, Pallisentis ophiocephali). From Polynemus paradiseus, a total of 10 species of parasites were recovered and identified. Among them, four were trematodes (Prosogonotrema bilabiatum, Uterovesiculurus hamati, Thaparotrema vittalani, Hypohepaticola callionymi); two cestodes (Nybelinia lingualis, Parachristianella trygonis); two nematodes (L4 larva of Dujardinascaris sp., Metaquimperia bagari) and two acanthocephalans (Neorhadinorhynchus aspinosum, Pallisentis ophiocephali). Acanthocephalan parasites showed the highest infestation rate (58%) whereas, no Cestoda was found in X. cancila. Trematode parasites showed the highest prevalence (68%) in P. paradiseus. The prevalence of parasites infestation was 60% in X. cancila (192 specimens) with mean intensity 1.14 per infested fish while in P. paradiseus, 49% was infested (158 specimens) with mean intensity 1.09. Regarding the organal distribution, most of the parasites were found to favour the intestine of both host fish. The prevalence of infestation in X. cancila was observed higher during winter season (November-February) while in P. paradiseus, it was higher during rainy season (July- October). The maximum intensity of parasites in X. cancila was recorded during the rainy season and in P. paradiseus, it was recorded during summer (March-June).
Keywords: Xenentodon Cancila; Polynemus Paradiseus; Helminths; Prevalence and Intensity
Fish is a very healthy source of protein for people in several places of the earth, specifically in developing nations. People have been consuming much aquatic animals to attain most of their protein requirements. Among the fresh water fish Xenentodon cancila (Hamilton Buchanan, 1822) is the only member belonging to Belonidae available in Bangladesh. And Polynemus paradiseus (Linnaeus, 1758) belongs to the family Polynemidae found in Bangladesh coastal and offshore waters and caught in large quantities from the shallow estuarine ground especially from Meghna, Chandpur and Bay of Bengal. The above mentioned two species are considered to be important fishes of Bangladesh because they are full of nutrients and delicious and have high market value. Fish perform an important role as a host for maintenance of helminth parasite. At the same time, fish is the host of several parasites and also act as a carrier of numerous larval parasitic forms that ultimately mature and cause severe illnesses in numerous terrestrial vertebrates including man (Schmidt, [1]). Fish face numerous parasitic agents and carrier hosts in their surroundings. Parasites are instinctively and functionally damaging for living beings and have predatory exploitative impacts, feeding on the host’s nutrients and causing distress in respiration. The demand of the infested fish in the market falls, resulting in large financial losses. The parasites may weaken and sometimes kill the fish by taking full advantage of their nutrients (Ekingen Zaman, Khanum, [2-3]). In Bangladesh, so far, research on the helminth parasites and biological aspects of fishes have been made mostly on edible fresh water and estuarine fish members under the families like Cyprinidae, Nandidae, Channidae, Anabantidae, Heteropneustidae, Notopteridae and Clupeidae. Only a few works have been done on the members of the family of Belonidae and Polynemidae.
The works on helminth parasites of Xenentodon cancila in Bangladesh have been done by (Bashirullah, Ahmed, Khanum, Sharmin, [4-7]). Altogether seven nematodes, viz. Camallanus gaboes, Camallanus xenentodoni, Contracaecum sp. Gnathostoma spinigerum, Paragendria bagarii, Metaquimperia bagarii, Procamallanus cancila; one trematode, Isoparorchis hypselabagri; and one acanthocephalan, Pallisentis ophiocephali were reported by them. (Khanum, [6]) worked on X. cancila and found 4 species of helminths. The prevalence was 24.88% with mean intensity 19.8. The only work on helminth parasites of Polynemus paradiseus in Bangladesh have been done by (Latifa, [8]). They studied the incidence of infestation of helminths parasites in Polynemus paradiseus and identified nine species of parasites of which four were trematodes viz. Prosogonotrema bilabiatum, Uterovesiculurus hamati, Thaparotrema vittalani, Hypohepaticola callionymi ; two cestodes, Nybelinia lingulalis, Parachristianella trygonis ; two nematodes, Dujardinascaris sp., Heterptyphylum cheni; one Acanthocephala, Neorhadinorhynchus aspinosum from the buccal cavity, oesophagus, stomach, intestine, caecum, liver, and body cavity of the host fish.
The ecological condition of rivers of Bangladesh has been changed after 1989. As no systematic study of the parasite of P. paradiseus and X. cancila has been done in our country, it was targeted to find out the diverse features of parasitic infestation and infection in the fishes.
A total of 321 Polynemus paradiseus and 321 Xenentodon cancila were autopsied and examined during January 2017 to December 2018, from Swarighat under Dhaka district of Bangladesh. The experiment was conducted at the Parasitology laboratory of the Department of Zoology, University of Dhaka. The sexes of each fish were identified according to (Haq [9]). The trematodes and cestodes were fixed in acetic-formalin-alcohol (AFA), stained in Semi chon’s aceto -carmine; cleared in lacto phenol and then mounted in DPX (Cable, [10]). The nematodes and acanthocephalan were fixed in Acetic acid, stained in borax carmine, cleared in lacto phenol followed by permanent mount on DPX (Cable [10]). For taxonomic classification of the helminth parasites, (Yamaguti, [11-14]) and other relevant reference articles were consulted.
In X. cancila (192 specimens), the prevalence of infestation of parasites was 60% with mean intensity 1.14 per infested fish while, in P. paradiseus, 49% was infested (158 specimens) with mean intensity 1.09 (Table 1). Out of 321 X. cancila examined, there were 178 male (55%) and 143 female (45%). On the other, out of 321 P. paradiseus, there were 148 male (46%) and 173 female (54%). In X. cancila, the prevalence in female (75%) was higher than male (48%) and in P. paradiseus the prevalence was slightly more in female (51.45%) than male (46.62%) (Table 2). In case of X. cancila, (Jan’17-Dec’17), a total of 160 fishes were examined and among them 91 were infected, the prevalence of infestation was 56.88% and the mean intensity of the parasites was 1.17 . In the next year (Jan’18-Dec’18), 161 numbers of host fishes were examined and 101 hosts were infected, the prevalence and the mean intensity of parasite were observed 62.73 % and 1.11 (Table 3). A total of 9 species of parasites were collected and identified from X. cancila, of which two were trematodes (Bolbocephalus sp, Isoparorchis hypselobagri); four nematodes (Metaquimperia bagari, L3 larva of Gnathostoma spinigerum, Camallanus ophiocephali, Porrecaecum trichuri.) and three acanthocephalans (Neoechinorhynchus prolixum, Acanthocentis nigeriensis, Pallisentis ophiocephali) (Plate 1, Figure 1). During Jan’17-Dec’17, a total of 161 P. paradiseus were examined and among them 85 were infected, the prevalence and the mean intensity of the parasites was 52.80% and 1.08. In the next year (Jan’18-Dec’18), 160 numbers of host fishes were examined, and 73 hosts were infected, the prevalence and the mean intensity of the parasites was 45.63 % and 1.10 (Table 3). From Polynemus paradiseus, a total of 10 species of parasites were recovered and identified.
Table 2: Prevalence and intensity of helminth parasites among P. paradiseus and X. cancila according to male and female.

Among them, four were trematodes (Prosogonotrema bilabiatum, Uterovesiculurus hamati, Thaparotrema vittalani, Hypohepaticola callionymi); two cestodes (Nybelinia lingualis, Parachristianella trygonis); two nematodes (L4 larva of Dujardinascaris sp., Metaquimperia bagarii) and two acanthocephalans (Neorhadinorhynchus aspinosum, Pallisentis ophiocephali) (Plate 2, Figure 2). In the present investigation, trematodes and nematodes were found to be dominant among the parasites (4 trematodes and 2 nematodes) in P. paradise; nematodes (4) and acanthocephalan (3) were found to be dominant in X. cancila (Table 4). In P. paradiseus and X. cancila, the parasitic fauna was observed to occupy the oesophagus, stomach, anterior and posterior intestine, body cavity, liver and the larval forms were found to be attached to the anterior intestine, liver and fat bodies. Regarding the organal distribution, most of the parasites were found to favor the intestine of both host fish. In X. cancila, the percentage of parasites present in different organs were: 46% in anterior intestine, 42% in posterior intestine, 5% in rectum, 4% in body cavity and 2% in liver (Figure 3a). In P. paradiseus, the percentage of parasites present in different organs were: 12% in stomach, 46% in anterior intestine, 36% in posterior intestine and 6% in liver (Figure 3b). Acanthocephalan parasites showed the highest infestation rate (58%) whereas no Cestoda was found in X. cancila (Figure 4). Trematode parasites showed the highest prevalence (68%) in P. paradiseus. Among the total helminth parasites recovered, the most numerically dominant and highly prevalent acanthocephalan was Pallisentis ophiocephali (23% with mean intensity 1.14) in X. cancila and 4% with mean intensity 1.17. In P. paradiseus , Prosogonotrema bilabiatum found 23% with mean intensity 1.03) (Figure 5).
Table 4: Index of similarities and dissimilarities of parasitic infestation in P paradiseus and X. cancila.
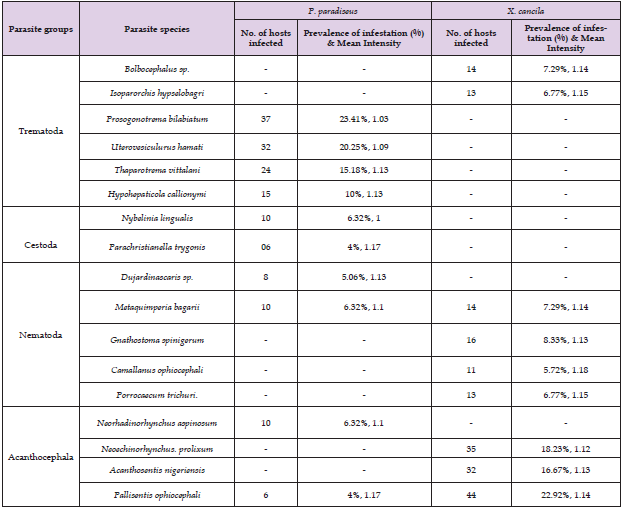
Figure 3:
a. Percentage of helminth found in various organs of Xenentodon cancila.
b. Percentage of helminth found in various organs of Polynemus paradiseus.

The present study showed that greater number of parasites have some special preference in their site selection to some extent. The changes occurred as the parasites become overcrowded in their niche (Mackiewicz, [15]). In the investigation, it was seen that the intestine of fish is usually infected more, probably because of the relatively abundant nutrient being present there. According to (Bullock, Dogiel, [16-17]), the intestine of fish is usually more infected than any other organ. In the present study, it was observed that Prosogonotrema bilabiatum, Uterovesiculurus hamati, Dujardinascaris sp., Metaquimperia bagarii, Neorhadinorhynchus aspinosum and Pallisentis ophiocephali were restricted to specific site or organ in P. paradiseus, but other species of parasites were not found in specific site within the same host fish. Whereas, juvenile P. trygonis in P. paradiseus was found to very specific in site preference. In X. cancila, juvenile Isoparorchis hypselobagri, Pallisentis ophiocephali were found to be more specific in site preference than the others. Some species of parasites occupied narrower microhabitat whereas others may be more flexible and occupied greater areas (Awachii , Mackenzie, Gibson, Ulmer, Holmes, Hine, Evans, [18-23]) concluded that the different site preferences of the parasites may be explained by innate variances among the parasite species which indicate their reactions to stimuli thus bringing them to be confined establishment and any successive migrations, influenced by some biochemical and physiochemical gradients in the different organs of the host. The exact reasons of seasonal variation in the infection of helminths depends on the aquatic invertebrate fauna and environmental factors eg. temperature, rainfall, PH and feeding habits and age of the host fishes cannot be explained easily (Khanum, [24]).
However, these changes can be traced back to diet and other factors like host size and growth of immunity which play a vital role in determining the incident (Scott, [25]). In X. cancila, seasonal abundances of total parasites showed distinct peak period of abundance (75% in Nov’18) during winter season (Figure 6). In P. paradiseus, it was clearly seen that during rainy season (remarkably in July’17) maximum number of parasites were found (Figure 7). Rainy and winter months were the most vulnerable period of the year when fish parasites were found plentiful. The reason for this could be stocking density, water depth, temperature, heavy rainfall, flood, various kinds of pollutants such as industrial pollutants, pesticides, insecticides, domestic sewage etc. and decreased immunity of hosts as well. This coincides with the findings of (Zaman, Khanum, [26,27]) where they agreed that the seasonal abundance of the helminth parasites are significantly correlated with the seasonal rainfall. The parasite which was dominant in a particular fish host, may or may not maintain its dominance in another host (Alom, S, Khanum, H, Khanum et al, [28- 29]). (Amin [30]) supports the view that the presence of a parasite species in significant number in a fish host, results in a lower density of the other species of parasites. It was evident that, prevalence and intensity of trematodes and nematodes varied greatly in both the years of the study period in both the host fishes. A few different species of parasites were also found in this investigation affecting the two hosts separately. Statistical analysis by “proportion test” showed that the overall proportion of infected P. paradiseus does not differ significantly at 5% level between two periods 2017 and 2018. A similar trend was also shown in X. cancila (Table 3).
Table 5: Association between male and female P. paradiseus and X. cancila (through chi square test).
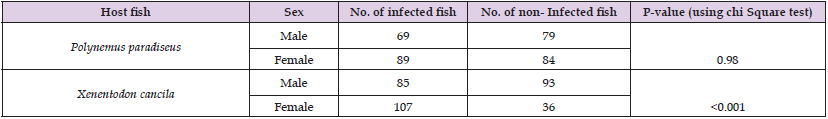
But the “chi square test” whether having association between number of infected fish and sex of the fish showed that there was no association between male and female P. paradiseus to be infected at 5% level of significance. Since P value is much smaller for X. cancila. The sex of X. cancila is statistically associated to be infected at 5% level of significance (Table 5). Thus, male and female have significant contribution of being infected in X. cancila. The host, the pathogen and the environment are in a constant state of instability, having the ability to change in any step with any variation may cause new infection of parasite in X. cancila and Polynemus paradiseus.
Special attention should be given to the inhibition of parasite infection. This will enhance the number of fish production. Sufficient knowledge regarding the control of parasites plays an important role in fish protection and production.


