Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ombugadu A1*, Ahmed HO1, Goler EE2, Abok JI3, Da’an SA4, Dogo KS1 and Markus M2
Received: October 03, 2023; Published: October 25, 2023
*Corresponding author: Ombugadu A, Department of Zoology, Faculty of Science, Federal University of Lafia, PMB 146, Lafia, Nasarawa State, Nigeria.
DOI: 10.26717/BJSTR.2023.53.008400
Heavy metals are an integral part of nature and play an important role in the lives of living species at trace levels. These become toxic when in excess in the organisms. Therefore, this study investigated the bioaccumulation of lead (Pb) in some birds and plant species in rapidly degrading mining sites in three selected Local Government Areas (LGAs) of Nasarawa State, Central Nigeria, from June 2017 to May 2018. Birds were trapped using mist nets. Ninety (90) tail feathers of birds and forty-eight (48) tree stand leaf samples were collected for quantitative measurements of Pb using the Atomic Absorption Spectrophotometer (AAS). A total of ninety (90) individual birds belonging to thirty five (35) species were trapped. Eighty-nine percent (89%) of the species were passerines while non-passerines were only 11%. The non-passerines accumulated more Pb than the passerines; however, there was no significant variation (t = 2.2312, df = 9.7714, P = 0.05034) between bird groups. Bird species-specific Pb bioaccumulation was highest in Shikra (3.24 ppm). The carnivorous birds had the highest Pb concentration, yet there was no significant difference (P > 0.05) in relation to the other feeding guilds. Pb concentration was not significant (P > 0.05) between birds’ age groups and sexes, respectively. The respective differences in mean Pb concentration in bird feathers as well as plant leaves across the surveyed sites significantly varied (P < 0.05). Ficus plant Pb concentration was highest over the other three plant species, although not significant (P > 0.05). The association in bioaccumulation of Pb between plants and birds was negative (t = -1.4223, df = 88, P = 0.1585, r = -0.15). The pooled average Pb bioaccumulation in birds as well as plants, respectively, was negligibly below the World Health Organization threshold (maximum permissible limit), which would have had an adverse effect. In conclusion, in the long term, Pb bioaccumulation might have more severe consequences for non-migratory species from the checklist of birds recorded in this study that carry out their entire life functions within a confined area. Thus, there is an urgent need for continuous bio-monitoring using this non-invasive technique, which will aid regulatory agencies in collaborating with miners and local communities to explore safer mining practices in order to conserve biodiversity in light of the detrimental effects of Pb bioaccumulation.
Keywords: Mining Sites; Lead Bioaccumulation; Birds; Plants; Atomic Absorption Spectrophotometer; WHO Maximum Permissible Limit; Nasarawa State
Abbreviations: ALAD: Aminolevulinic Acid Dehydratase; LGAs: Local Government Areas; AAS: Atomic Absorption Spectrophotometer; MPL: Maximum Permissible Limit
The tremendous quest for development through industrialization and urbanization in the past few years has led to a series of environmental contaminations from heavy metals [1]. Among the toxic metals found in the earth’s crust, lead (Pb) has a more widespread use, resulting in serious environmental contamination, human, plant, and animal exposure, and significant public health problems in many nations of the world [2]. Mining and smelting of Pb have significantly stood out as an important source of environmental contamination amidst manufacturing and recycling activities. Environmental Pb contamination has also been reported in some countries through the continuous use of leaded paints and leaded gasoline [3]. Apart from releasing other air pollutants, including sulphur dioxide and nitrogen oxides, in addition to leaving behind tons of waste tailings, slag, and acid drainage, mining sites are responsible for some of the largest releases of toxic heavy metals, including Pb, into the environment [3]. It has been reported that Pb is the second most hazardous substance after arsenic based on its frequency of occurrence, toxicity, and exposure by the Agency for Toxic Substances and Diseases Registry [4-6]. Pb contamination in the soil could take many years, and its effect on both plants and birds remains a bigger problem. For example, mining activities in Tri-State Mining Districts, Kansas, Oklahoma, and Missouri, USA, were suspended as far back as the 1970s, yet toxic metals like Pb still remain at high concentrations in mine waste and sediments [7,8]. Plaza, et al. [9], in their attempt to compile existing knowledge about Pb contamination with respect to South American bird species, were able to point out that there is little information about Pb contamination and the species of birds affected. The most predominant source of Pb contamination for wild birds remains the spent Pb ammunition since birds would pick and swallow it; nevertheless, contaminations from mining sites also have significant effects and affect a great variety of birds, leading to death, a decline in population, and biodiversity loss [9,10].
Pb has no known biological requirement and acts as a non-specific poison affecting all body tissues and systems of a significant number of wild birds [11]. For over a century, high Pb exposure in birds has been a concern, as cited in studies by Perez-Lopez, et al. [12-14]. Some reports have been made on the toxicity nature of Pb on bird species, including waterfowl, upland birds, raptors, and scavengers, as noted by Plaza, et al. [9,15-17]. The toxicity in birds starts with the ingestion of Pb-contaminated plants, water, and even earthworms, as in the case of an investigation carried out on songbirds [18-20]. The digestion process then leads to the absorption of Pb into the guts and then into the bloodstream, where it is transported to all organs and tissues of the bird. Toxicity in birds can range from simple hematologic changes to even serious and lethal pathologic alterations depending on the level of exposure with millions of birds recorded to die annually as a result of lead poisoning all around the world [9,10,21,22]. Even though Pb toxicity and health effects on birds can vary depending on the level of concentration and exposure or even differences in gastrointestinal physiology, environmental temperature, and diet composition, some common symptoms and effects include clinical changes like weakness, weight loss, diarrhea, incoordination, anemia, reproductive changes, immune system dysfunctions, behavior changes, changes in migratory patterns, and bone mineralization.
The Tri-State Mining Districts of Kansas, Oklahoma, and Missouri, USA, have recorded significant Pb poisoning in migrating waterfowl species, Canada Geese (Branta canadensis), who are particularly attracted to the mining habitat due to open water and food sources since they can tolerate human activities and are often attracted to suburban and agricultural environments. These Canada geese showed a high tissue concentration of Pb, leading to physiological indications of Pb exposure in the form of inhibited blood aminolevulinic acid dehydratase (ALAD) activity [8]. It can only be concluded that the Canada Geese consistently suffered adverse health effects associated with Pb exposure from these mining sites. Ombugadu, et al. [23] opined that the average Pb concentration in birds in a polluted site (i.e., the Kaduna Refinery Area in Kaduna State) was about two times higher than in the protected Amurum Forest Reserve area in Jos, Plateau State, Nigeria.
Plants, among other living organisms, are the most targeted by a wide range of pollutants since they are stationary in nature, which results in their exposure to soil and atmospheric toxicants [24-26]. Ombugadu, et al. [23] posited that heavy metals are deposited on plant surfaces. Lead is a major pollutant and contaminant of plants, and its sources range from smelting and mining activities to the combustion of Pb fuels from motor engines to even the use of Pb-formulated fertilizers on farmlands [25,27-30]. The main route for Pb uptake in plants has been through the roots [25,31,32]. From there, Pb is adsorbed onto the roots of the plants to become bound to the carboxyl groups of mucilage uronic acid or even directly attached to the polysaccharides of the rhizoderm cell surface of the roots [25,33]. From the root rhizoderm, the Pb may enter the roots passively and into the trans-locating water streams to be transported to the other parts of the plants [25,34,35]. Several plant species, including Vigna unguiculata [25,36], Festuca rubra [25,37], Brassica juncea [25,38], Lactuca sativa [25, 32], and Funaria hygrometrica [25,39] have indicated Pb adsorption onto their roots according to documented reports. Exposure of plant seedlings to concentrations of Pb, even at lower levels, can greatly inhibit the rate of germination. Pourrut, et al. [25,40-42] have reported Pb-induced inhibition of seed germination in Hordeum vulare, Elsholtzia argyi, Spartina alterniflora, Pinus halepensis, Oryza sativa, and Zea mays.
It has also been explained that at high concentrations, Pb contamination may speed up seed germination but in turn induce adverse effects on the length of the radical and hypocotyl in Elsholtzia argyi [25,41]. Pb toxicity impairs plant growth, root elongation, seed germination, seedling development, transpiration, chlorophyll production, a lamellar organization in the chloroplast, and cell division, even though these effects may vary depending on the concentration of Pb in the soil, the duration of exposure, and the stage of development of the particular plant in question [25,28,31,39,43,44].
In Nigeria, the disposal of natural gas by flaring is widely practiced, with no available data on the extent of metal pollution associated with this wasteful practice [23]. In 2010, the illegal excavation of soil contaminated with Pb for gold in Zamfara State, Nigeria, resulted in Pb poisoning, which killed over 355 more people than the whole world combined records from 1970 to 2010 [45]. Illegal mining activities are known to be going on in many parts of Nasarawa State [46], resulting in the release of mineral dust that contains heavy metals and is being deposited on plants and animals [47]. A large number of miners in Nasarawa State pay little or no attention at all to environmental considerations. Most mining activities carried out in the state are done in a way that will not safeguard the lives and fortunes of future generations. Also, there is a paucity of information (knowledge gap) on the bioaccumulation of Pb in resident birds and plants in mining sites within Nasarawa State. An ecological study of this magnitude will assess the impact of mining activities (environmental degradation), which will be a reflection of Pb level in the environment and biomarker of accumulation in humans. To this end, the bioaccumulation of Pb in some birds and plant species in rapidly degrading mining sites in three selected Local Government Areas (LGAs) of Nasarawa State, Central Nigeria, was explored non-invasively so as to quantify Pb level in order to proffer valuable solutions that are sustainable and environmentally friendly for the wellbeing of all.
Study Areas
The study was carried out in mining sites in Awe, Lafia, and Nassarawa Eggon LGAs of Nasarawa State, Nigeria, as shown in Figure 1. Nasarawa State is endowed with solid minerals in the Guinea-Savanna eco-region of Nigeria. The state has a land mass of 27,862 km2 and a population of 1,863,275 people [46]. Heavy mining activities are ongoing in the three (3) selected LGAs, as captured in the pooled images in Plate 1, and are becoming an alarming and rapid environmental degradation signal. The mining sites are characterized by vegetation as well as water bodies in which water from mining pits is being discharged into flowing water bodies, thereby making them unsafe for aquatic organisms and the communities around them (Plate 2).
Duration of Study
This study was carried out from June 2017 to May 2018.
Samples Collection
Sampling of Birds: To collect feather samples, ten 20-meter mist nets (Plate 3) were used to trap birds at the study sites. The mist nets were opened and closed in the morning between 5:30 a.m. and 9:30 a.m. All birds trapped were identified and grouped according to their feeding guilds, age, sexes, and ringed [23,48,49]. For each trapped bird, the second outer tail feather from the left was collected, placed in a well-labeled envelope, and taken to the laboratory [23].
Sampling of Plants: The plant species selected for this study include Dichrostachys cinerea (Syn: D. glomerata: Leguminosae: Mimosoideae), Ficus species, Parkia biglobosa, and Mangifera indica, which are widespread in tropical Africa and common in savanna regions, where they grow as large shrubs and are sources of food, fodder, and medicine to the indigenous communities there [50]. Sampling was done as described by Ombugadu, et al. [23]. Four twigs were collected about 1-2 meters from the base of the crown of each of the four tree species. Each tree species had four replicates. The replicate of each tree species was collected from the four cardinal directions (North, South, East, and West) to capture possible variation due to changes in wind directions around the mining area. Ten fully expanded leaves were removed from the tip, middle, and basal parts of each twig, and the samples were bulked for each tree (i.e., 30 leaves per tree). For each species, a total of 120 fully expanded leaves were sampled. Samples were sealed in clean polythene bags and taken to the laboratory.
Sample Preparation and Chemical Analysis for Lead (Pb) in Birds and Plants
Birds’ feather were prepared and digested directly, whereas plant leaves first went through an ashing phase before they were digested. Prior to the processing of samples, all glassware and plastics were washed, rinsed many times with tap water, and then soaked in a 5% nitric acid solution for 24 hours, followed by rinsing with deionized water. Analytical grades of nitric acid (65%, Sigma Aldrich) and perchloric acid (70%, Sigma Aldrich) were used for sample preparation. The standard solution for calibration of Pb was prepared from a 1000 mg/L standard stock solution of GFS Fishers’ Atomic Absorption Spectrophotometer (AAS) Reference Standard. All the solutions were prepared with distilled water. The dilution correction method was applied to samples diluted or concentrated during analysis.
Birds Tail Feathers Processing: A pool of ninety (90) birds’ tail feather, thirty from each LGA, were processed and analyzed for the presence of Pb. Feathers were washed three times using distilled water alternated with acetone in order to remove possible contaminants [23,51]. The samples were then air-dried before oven drying at 105°C for 2 hours. The feathers were subsequently cut into smaller pieces using stainless steel scissors to facilitate the acid digestion process. The acid is a mixture of concentrated hydrochloric acid (HCl4) and nitric acid (HNO3) in a ratio of 3:1 (i.e., 400 ml to 133 ml) by volume (aqua regia). One gram (1 g) of each feather sample was added to the acid mixture for complete digestion. The digestion was stopped when a colorless solution was obtained. The resulting solution was diluted to a volume of 25.0 mL with deionized water. The solutions were allowed to cool, then filtered using Whatman filter paper into a 100 ml calibrated flask and diluted up to the mark. Thereafter, each sample solution was analyzed for Pb using a flame AAS.
Plants Leaves Processing: The 48 individual plant stands from four plant species leaf samples were washed carefully in running tap water and rinsed twice with distilled water. These were oven-dried at 65°C for 48 hours in labeled, large paper envelopes [23,50]. Dried samples were ground to a fine powder in an agate mortar. For each sample, 0.5 g of the ground fine powder was placed in a crucible and ashed at 550°C in the muffle furnace. Thirty (30) ml of aqua regia was added to each ashed sample, which was centrifuged and filtered into well-labeled, clean sample bottles. The filtrates were then analyzed for Pb using an AAS machine.
Maximum Permissible Limits of Pb in Birds and Plants
Pb detected in birds was compared to the World Health Organization maximum permissible limit (WHO MPL) of 2 ppm, as described by Pain, et al. [52]. Also, the 7 ppm WHO MPL standard for plants, as cited in Oyewo, [53], was used for comparison with the Pb values detected in plants surveyed.
Statistical Analyses
The R-console software (version 4.0.2) was used for statistical analyses. The Shapiro-Wilk-normality test was used to determine the normality of the data distribution. Welch’s two-sample t-test was used to compare the bioaccumulation of Pb between bird groups. One-way analysis of variance (ANOVA) was used to compare Pb concentration in relation to bird species, feeding guilds, plant species, and surveyed sites, respectively. The differences in the mean Pb concentration between the ages and sexes of birds, respectively were analyzed using student’s t-test. Pearson’s product-moment correlation was used to determine the association between Pb concentrations in plants and birds. The level of significance was set at P < 0.05.
Concentration of Pb Metal Between Passerines and Non-Passerines in Selected Mining Sites
Of the thirty-five (35) bird species analyzed for bioaccumulation of Pb, the passerines were made up of 28 species which comprised 80 individuals (89%) while non-passerines had 7 species with 10 individuals (11%). No significant variation (t = 2.2312, df = 9.7714, P = 0.05034, Figure 2) was observed in the bioaccumulation of Pb between bird groups, although on the average non-passerines accumulated a higher amount of Pb than the passerines. This may be a result of their body mass as well as the hierarchy of the non-passerines in trophic level. This agrees with the study by Sani [54] who recorded very high Pb concentration in the feathers of non-passerines trapped in the Kano metropolis around industrial, residential, and commercial areas. On the contrary, a study on the concentrations of toxic metals in feathers shows that passerine birds collected from four regions of North-Eastern Pakistan had a high concentration of Pb [13]. Also, a very high amount of Pb concentration was found in Passer montanus (tree Sparrow) a free-living resident passerine bird in two regions of China [14].
Species-Specific Pb Concentration
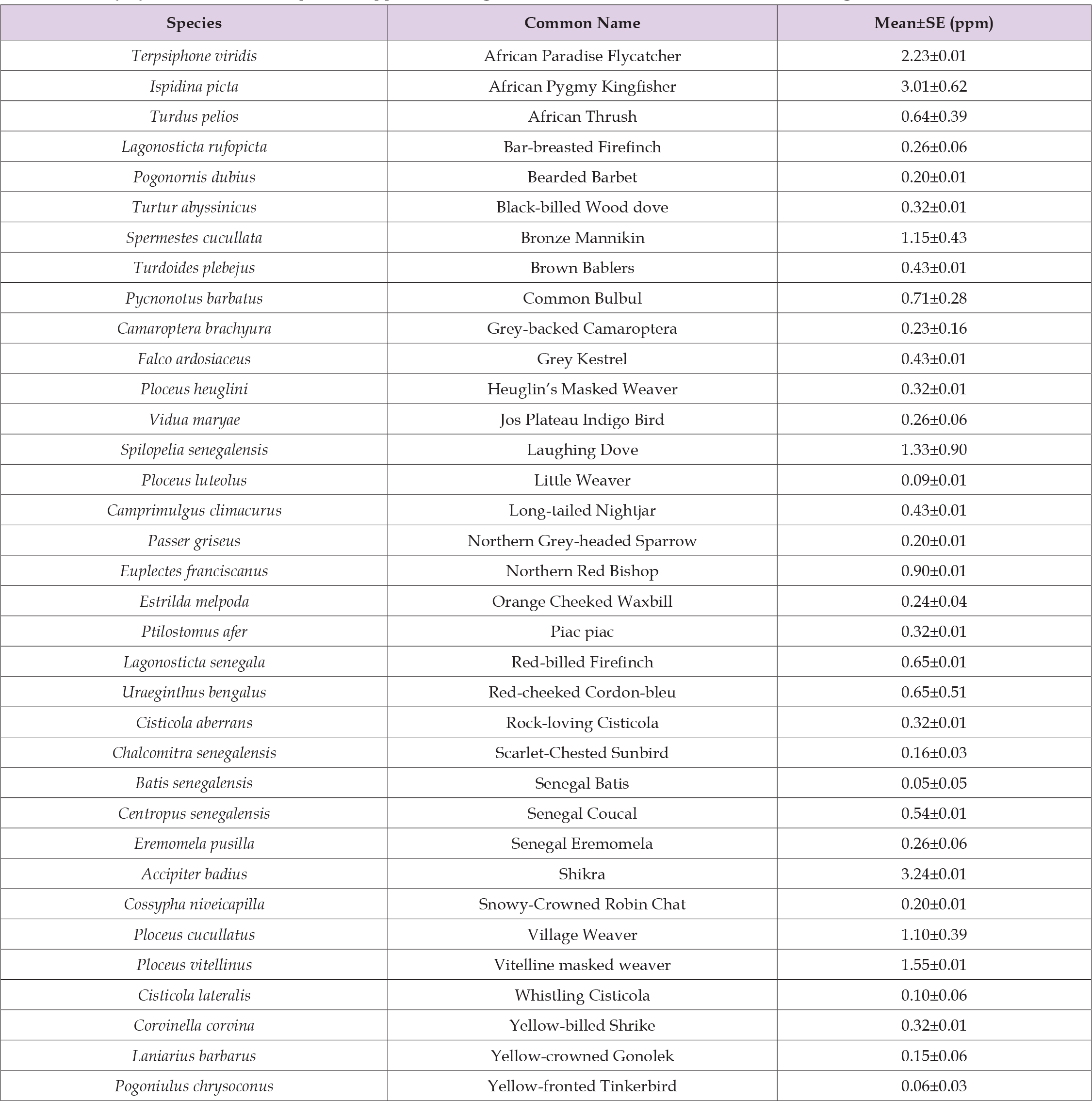
Bioaccumulation of Pb between bird species showed no significant difference (F55 = 1.478; Adjusted R2 = 0.1545, P = 0.09685). This is not in line with the findings of Aziz, et al. [13], who reported differences in Pb concentration among bird species. Similarly, Gushit, et al. [55] observed variations in Pb concentration among birds in an urban degraded woodland in Jos, Plateau State, Nigeria. Although bird species-specific bioaccumulation of Pb was highest in Shikra at 3.236 ppm, followed by African Pygmy Kingfisher at 3.011 ppm, African Paradise Flycatcher at 2.225 ppm, and Senegal Batis had the lowest concentration of 0.045 ppm, as shown in Table 1. The high concentration of Pb in Shikra birds may possibly be due to their position in the trophic level (type of food) and the systematics of the sampled species. This present study agrees with the finding of Durmus [56], which showed that members of the Accipitridae family highly accumulated heavy metals in the Van Lake Basin in Turkey. The Shikra bird in this study belongs to the family Accipitridae, which is worthy of note.
Table 1: Lead (Pb) level in some bird species trapped in mining sites in 3 selected LGAs in Nasarawa State, Nigeria.
Pb Accumulation in Birds in Relation to Sites
The presence of Pb in the environment highly accumulated in birds from Lafia area (1.62±0.23 ppm) followed by those trapped in Nassarawa Eggon LGA (0.24±0.03 ppm), while it was very low (0.23±0.04 ppm) in birds in Awe LGA. Thus, bioaccumulation of Pb between surveyed sites significantly varied (F87 = 35.92, Adjusted R2 = 0.4397, P < 0.0001, Figure 3). The differences across the sites may most likely be a result of varying mining activities and other pollutants in the Lafia environs. This agrees with Aziz, et al. [13], who reported significant differences in Pb concentration across four study regions of north-eastern Pakistan. Correspondingly, Ombugadu, et al. [23] found significant variation in Pb concentration between the protected Amurum Forest Reserve in Jos-East, Plateau State, and the Nigerian National Petroleum Corporation (NNPC) Refinery Area in Kaduna State, Nigeria. Also, the concentration of Pb varied between the polluted site (near a metallurgic factory) and the control site as documented by Dauwe et al. [47] and O’Meara et al. [57].
Age and Sex-wise Concentration of Pb in Birds
Juvenile birds had a higher concentration of Pb (0.71±0.386 ppm) than adults’ (0.70±0.11 ppm). However, age-wise differences in the mean concentration of Pb was not significant (t = -0.033439, df = 88, P = 0.9734, Figure 4). The lack of variation across age groups possibly suggests that the feather of a bird of any age could play the role of a bioindicator. This is per the studies by Ombugadu et al. [23] and Dauwe et al. [47], who reported no age-related difference. However, Ding, et al. [14] observed a significant difference in Pb concentration between the feathers of juvenile and adult birds.
Pb concentration was higher in male birds 0.92±0.22 ppm, while in females it was absolutely less by half 0.45±0.12 ppm, and differences was not significant (t = -1.8927, df = 38.302, P = 0.06597, Figure 5). The absence of variation in Pb concentration between the sexes probably suggests that either of the sexes can serve as an environmental bioindicator. This corresponds with previous findings by Ombugadu et al. [23], Dauwe et al. [47], Scanlon et al. [58], and Devkota and Schmidt [59] that found evenness in Pb concentration for both sexes. However, it does not concur with the recent study by Ding, et al. [14], who observed differences in Pb concentration between male and female birds.
Pb Bioaccumulation in Birds to Feeding Guilds
Of the six feeding guilds recorded in this study, the concentration of Pb was very high in carnivorous birds (1.83±1.40 ppm), followed by granivores (0.83±0.16 ppm), and generalists (0.71±0.28 ppm), whereas a very low Pb level was observed among frugivores (0.10±0.04 ppm). Nonetheless, the differences in Pb concentration between the six feeding guilds were not significant (F84 = 1.381, Adjusted R2 = 0.02093, P = 0.2399, Figure 6). This is not in agreement with the finding of Durmus [56], whose evaluation revealed variation in heavy metal concentration across bird feeding guilds, in which the carnivorous birds had the highest heavy metal concentration. Similarly, Ombugadu, et al. [23] reported a remarkable difference in Pb concentration among feeding guilds of birds within the Amurum Forest Reserve in Jos, Plateau State.
Concentration of Pb in Some Plants in Selected Mining Areas
From the four plant species analyzed, Ficus species had the highest Pb concentration of 0.44±0.14 ppm, followed by Parkia biglobosa (0.32±0.05 ppm), Mangifera indica (0.23±0.06 ppm), and Dichrostachys cinerea (0.22±0.05 ppm). Yet, there was no significant difference (F44 = 1.293, Adjusted R2 = 0.01839, P = 0.2886, Figure 7) in Pb concentration among the four plant species in mining sites. The lack of variation in Pb concentration between the four plant species may possibly be due to their stationary nature or form, which makes them easily exposed and vulnerable to the equal amount of Pb dust being released into the atmosphere from mining sites. On average, Ficus plants had a very high Pb concentration, which agrees with Alaimo [60], who confirmed that Ficus macrophylla leaves are suitable for screening an urban environment to identify concentrations of inorganic chemicals due to their high tolerance to pollution. On the contrary, previous studies by Ombugadu, et al. [23] showed that Mangifera indica accumulated Pb the most, while Ficus platyphylla had the least amount of Pb.
The differences in Pb concentration in plants in relation to the sites surveyed was highly significant (F45 = 7.635, Adjusted R2 = 0.2202, P = 0.001396, Figure 8), although the plants from the Awe area had the highest concentration of Pb (0.52±0.10 ppm), followed by those from Nassarawa Eggon (0.21±0.04 ppm), while it was low in Lafia (0.18±0.04 ppm). The strong variation in Pb accumulation between plants surveyed from the three LGAs in this study is not in line with the finding of Ombugadu, et al. [23], who recorded relatively equal Pb concentration between the Kaduna Refinery Area and the protected Amurum Forest Reserve area.
Association of Pb Level in Plants and Birds
The relationship between the bioaccumulation of Pb between plants and birds was negative (t = -1.4223, df = 88, P = 0.1585, r = -0.15, Figure 9). The sharp negative association of Pb concentration between plants and birds probably suggests that Pb does not directly accumulate from plants to birds; rather, it follows the gradual buildup along the regular food chain process as well as trophic level energy flow.
Mean Concentration of Pb in Comparison with WHO Maximum Permissible Limits in Birds and Plants
Figure 10 shows the amount of Pb in birds and plants compared to the WHO maximum permissible limit (WHO MPL) and the average of all the recorded values for each. The levels of Pb in birds and plants were all below the WHO maximum permissible limit of 2 ppm [52] and 7 ppm [53], respectively. This possibly suggests that Pb concentration is yet to exceed the threshold level to elicit adverse effects on the well-being of plants, birds, and, in the long run, higher animals (humans) who may consume them, thereby resulting in many negative effects on human bodies such as damaging kidneys, the nervous system, and the reproductive system. The recorded concentration of Pb in birds varied from 0.05 ± 0.05 to 3.24 ± 0.00 ppm in the present study. A few of the birds had Pb concentrations higher than the WHO MPL, which is clear-cut evidence of bioaccumulation. The Pb range in this study is similar to previous work by Gruz, et al. [61] whose Pb concentration ranged from 1.15 to 2.3 ppm. An analysis of Pb from different feathers in Calamus and Vane by Yamac, et al. [62] reported a range value of 0.65 ppm and 5.47 ppm, respectively, which is relatively higher than in our study. The reason for the lower concentration in this study is likely due to the local mining techniques used in these areas.
This study shows a relatively high concentration of Pb in some birds, which is above the WHO MPL is a good index of the health of the three environments surveyed and may have lethal implications, as Pb concentration in feathers (specifically tail feathers) has been observed in other studies to correlate significantly with concentrations in internal tissues and other feathers. Lead pollution in birds in the area is neither age nor sex-specific. Although Pb concentration in males was much more twice the amount in females. A significant variation was observed in the bioaccumulation of Pb in relation to the three mining sites. Hence, the use of non-invasive approaches such as collection of birds’ tail feathers and plant leaves, respectively, should be considered in biomonitoring the health of mining sites and environs. Finally, inhabitants of mining sites should plant more trees given that they have a well-established ability to absorb, detoxify, and withstand higher concentrations of heavy metal pollution that may have an adverse effect on biodiversity, the environment, and human health.
Conflict of Interest
All the authors declared no conflict for the publication of this article.
Acknowledgement
This work was supported by the Tertiary Education Trust Fund (FUL/VC/TETF/010/VOLI/IBR) and the Management of the Federal University of Lafia. Also, we are most grateful to the miners and surrounding communities who granted us access during the course of this study.


