Abstract
TaffiX® is a personal anti-viral nasal powder that is commercially available in Israel and Europe. To test if TaffiX® was able to form a protective barrier against SARS-CoV-2 gels of TaffiX® were formed on nylon filters, and then seeded with SARS-CoV-2. After 10 minutes the bottom of each filter was washed and tested for live virus by plaque assay and viral RNA using qRT-PCR. TaffiX® blocked SARS-CoV-2 in vitro, reducing the titer of live virus and viral RNA by 99%, supporting the use TaffiX® as a barrier against SARSCoV- 2 that could be used in conjunction with other protective measures.
Keywords: Infectious Diseases; Viral Fever; Quantitative Research; SARS-CoV-2; COVID-19
Introduction
Effective measures that reduce exposure to the SARS-CoV-2
virus are an important aspect of containing the COVID-19
pandemic. With uncertainties surrounding the emergence of new
variants and vaccine protection, and evidence that presymptomatic
and asymptomatic transmission of SARS-CoV-2 are significant
factors contributing to spread of infections [1], there is a need for
mechanisms that can reduce exposure to the virus. The cellular
entry of SARS-CoV-2 depends on the binding of the spike (S) protein
to a specific cellular receptor, angiotensin-converting enzyme 2
(ACE2), and subsequent S protein processing by cellular proteases
[2]. ACE2 and other proteases are highly expressed in ciliated and
goblet nasal cells, which supports the growing understanding that
nasal infection is the dominant route of transfection for COVID-19
viruses and therefore protection of the nasal epithelium is an
effective means of controlling infection [3,4].
Hou et al. describe the “infection gradient” whereby SARSCoV-
2 shows a gradient infectivity from the proximal to distal
respiratory tract, with ciliated airway cells and AT-2 cells as
primary targets for SARS-CoV-2 infection [5]. The authors conclude
that the nasal epithelium is an important gateway and therefore its
protection could dramatically decrease the risk of viral infection
and transmission. TaffiX®, a nasal hydroxypropyl methylcellulose
(HPMC) based powder spray, is a semisynthetic, inert, viscoelastic
muco-adhesive polymer used frequently in the pharmaceutical
industry. HPMC is used in eye drops, as an excipient and controlleddelivery
component in oral medications, and found in a variety
of commercial products including several nasal powder products
used as prevention of allergy [6,7]. Polymer-based powder
formulations show no adhesion until their absorption of mucus
occurs on the nasal mucosa surface. This allows easy application to
the nasal cavity by nasal insufflation even if the polymer is highly
mucoadhesive [8].
TaffiX® creates an acidic gel (pH 3.5) once it has reached the
nasal mucosa thus creating a local hostile microenvironment to
viruses at a pH range known to be associated with viral death [9].
TaffiX® also contains benzalkonium chloride which is known as an
antimicrobial preservative and was shown by Chin et al. to inactivate
SARS-CoV-2 [10]. This study we report that TaffiX® was able to form
a protective barrier against SARS-CoV-2 virus using experimental in
vitro conditions that reduced the titer of recoverable live virus, as
well as viral RNA by 99%.
Materials and Methods
TaffiX® Assay
One hundred-fifty μl of sterile water was added to individual sterile 40 μm cell strainers (BiologGLX) in the wells of a 6 well plate. Twenty mg TaffiX® was added and mixed with the water with a pipet tip until homogeneous across the cell strainer surface, and incubated at room temperature for 10 min. Ten μl of virus, (10,000 PFUs of Hong Kong/VM20, BEI Resources), was added to the center of each cell strainer and incubated at room temperature for another 10 min. The bottoms of the cell strainers were washed with 500 μl serum-free DMEM and flow through was collected for RNA isolation and plaque assays.
Plaque Assay
Plaque assays were performed as described in [11]. Vero C1008, Clone E6 (ATCC CRL-1586) cells were grown in DMEM (GIBCO 11995-040) and then seeded into 12-well tissue culture plates at a concentration of 2x105 cells/well the night before the assay. Viral stocks were diluted in cold serum free DMEM and added to the wells. Plates were incubated at 37°C, 5% CO2 for 2 hrs, and swirled every 15 min. After 2 hrs the media was replaced with 1.5 ml of 1X DMEM, 2.5% FBS, 1.2 % Avicel PH-101 (Sigma Aldrich) and incubated at 37°C, 5% CO2. After 2 days the Avicel was removed and the wells were washed with 1 ml of PBS. Ten percent formaldehyde was added. After 1 hr wells were washed with PBS, and then 0.1% crystal violet was added for 30 minutes and then washed with water.
Quantitative Reverse Transcriptase PCR (qRT-PCR)
RNA was isolated from 200 μl of the flow through wash from the Taffix® assay using QIAamp Viral RNA Mini Kit (Qiagen) according to manufacturer’s instructions. The PCR reaction consisted of UltraPlex 1-Step ToughMix (Quantabio) and a combined primer/ probe mix 2019-nCov_RUO kit (Integrated DNA Technologies). The positive control was 2019-nCoV_N Positive Control. The reaction conditions were 50°C for 10 min, 95°C for 3 min, and then 45 cycles of 95°C for 20 sec, 55°C for 30 sec.
Taffix® Gel formation in Pig Noses
The Taffix® powder (4 puffs per nostril, parallel to 1 puff per human nostril as indicated in product instructions) was sprayed on a fresh slices of pig nasal cavity, obtained from a meat processing plant, which was then soaked in saline and then placed in an oven at 34°C to resemble the human nose conditions and clinical dose per surface area. A blue color (Instacoat Color Blue, Ideal Cures Pvt. Ltd.) was mixed with powder before spraying for visualization. The gel formation time, and appearance were noted. The pH was measured with pH-FIX sticks (range 3-6, Macherey-Nagel) for 5 hours.
Results
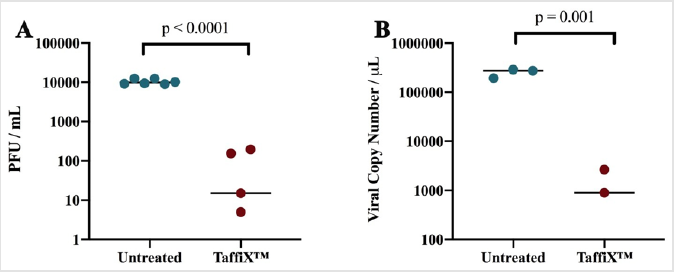
To test whether the TaffiX® can form a protective barrier against SARS-CoV-2, a gel of TaffiX® was formed on a 40 μm nylon filter using an amount equivalent to a clinical dose of 5 mg per nostril, the recommended usage dose. 10,000 PFUs of SARS-CoV-2 was applied to the TaffiX® gel-treated filter; the same amount of virus was added to an untreated filter as a control. After a 10 min incubation, the bottom of each filter was washed and the resulting flow through tested for live virus by plaque assay and viral RNA using qRT-PCR. TaffiX® reduced the amount of infectious virus in the flow through by more than 99% (Figure 1A), with an average three log reduction in titer. The number of copies of the viral genome recovered was also decreased by 99% by TaffiX® treatment as determined by qRTPCR (Figure 1B), with an average drop in copy number of over two logs, although detection of viral RNA does not necessarily represent infectious virus.
Figure 1: TaffiX® treatment reduced the amount of infectious SARS-CoV-2 virus. A) Viral titers of live virus in the flow through as determined by plaque assay. B) Copies of viral RNA as determined by qRT-PCR in TaffiX®-treated and untreated SARSCoV- 2 flow through. P values were determined by two tailed Student’s T test. Data represents 6 (plaque assays) and 3 (qRTPCR) biologically independent experiments. One TaffiX treated sample in panels A and B had no detectable virus or viral RNA so they are not represented.
To test the stability of the gel pH on nasal tissue ex vivo, the powder was sprayed on a fresh slices of pig nasal cavity soaked in saline solution and placed covered in an oven at 34°C to resemble the human nose conditions and clinical dose per surface area. The gel formation time, and gel appearance during 6 hrs were monitored as well as gel pH. TaffiX® powder formed a uniform gel on a pig nasal cavity tissue within 1 min from spray and was stable as a gel film for 6 hrs. The initial pH of the gel was 3.6 and the final (after 6 hours) pH was 4.4.
Discussion
Under in vitro conditions, TaffiX® formed a protective barrier against SARS-COV-2. Although these are artificial conditions, ex vivo experiments with pig nasal tissue demonstrated that a TaffiX® gel is formed within 1 minute from spray and an acidic film is maintained on the tissue for at least 5 hours. Without the gel protection, the human nasal mucosal pH is approximately 5.5-6.5 [12], which is a comfortable environment to SARS-CoV-2 virus. Clinical study by Hull et al. demonstrated in a randomized, double blind, placebocontrolled study that irrigation with an acidic nasal hydrogel spray reduced the severity and duration of the common cold symptoms [13]. TaffiX® is a safe and easy to use powder that may provide a straightforward mechanism to reduce infections and thus the spread of COVID-19.
Declaration of Competing Interest
T. Lapidot, PhD and D. Megiddo, MD are employees of Nasus Pharma.
Funding
This work was supported by Nasus Pharma, Tel Aviv, Israel.
Footnote
TaffiX® is approved in Israel (Amar: 33010001) with the following Indication for Use: TaffiX® is intended for use to block inhaled viruses and bacteria within the nasal cavity. TaffiX® is legally marketed in Europe (CE- DE/CA09/0760/N18/001) with the following Indication for Use: TaffiX® is indicated for use as a protective mechanical barrier against allergens and viruses (e.g., SARS-CoV-2) within the nasal cavity.
References
- Furukawa NW, Brooks JT, Sobel J (2020) Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic. Emerg Infect Dis 26(7): 201595.
- Wan Y, Shang J, Graham R, Baric RS, Li F (2020) Receptor Recognition by the Novel Coronavirus fromWuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol 94(7): 127-130.
- Ziegler CGK, Allon SJ, Nyquist SK (2020) SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 181(5): 1016-1035.
- Sungnak W, Huang N, Bécavin C (2020) SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 26(5): 681-687.
- Hou YJ, Okuda K, Edwards CE (2020) SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 182(2):429-446.
- De Silva DJ, Olver JM (2005) Hydroxypropyl methylcellulose (HPMC) lubricant facilitates insertion of porous spherical orbital implants. Ophthal Plast Reconstr 21(4): 301-302.
- Williams RO, Sykora MA, Mahaguna V (2001) Method to recover a lipophilic drug from hydroxypropyl methylcellulose matrix tablets. AAPS PharmSciTech 2(2): 29-37.
- Vidgren MT, Kublik H (1998) Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev 29(1-2): 157-177.
- Lamarre A, Talbot PJ (1989) Effect of pH and temperature on the infectivity of human coronavirus 229E. Can J Microbiol 35(10): 972-974.
- Rennie P, Bowtell P, Hull D, Charbonneau D, Lambkin-Williams R, et al. (2007) Low pH gel intranasal sprays inactivate influenza viruses in vitro and protect ferrets against influenza infection. Respir Res 8: 38.
- Baer A, Kehn-Hall K (2014) Viral concentration determination through plaque assays: Using traditional and novel overlay systems. J Vis Exp 93.
- England RJA, Homer JJ, Knight LC, Ell SR (1999) Nasal pH measurement: A reliable and repeatable parameter. Clin Otolaryngol Allied Sci 24(1): 67-68.
- Hull D, Rennie P, Noronha A (2007) Effects of creating a non-specific, virus-hostile environment in the nasopharynx on symptoms and duration of common cold. Acta Otorhinolaryngol Ital 27(2): 73-77.

 Research Article
Research Article